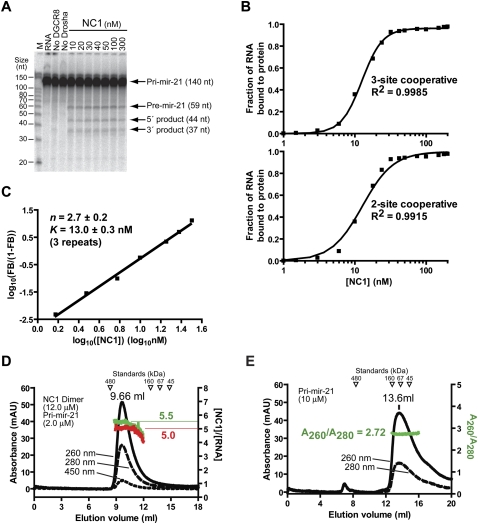
FIGURE 3.
Processing of pri-mir-21 in a DGCR8-dependent fashion and trimerization of DGCR8 upon association with pri-mir-21. (A) Reconstituted pri-mir-21 processing assays. The reactions were incubated for 45 min at 37°C. Recombinant Drosha protein (10–20 nM) was present in all reactions, except the “no Drosha” sample, which only contained 100 nM of NC1. (B) Results of the filter binding assays. The upper panel indicates the fitting of the data using a cooperative trimer model, in which three NC1 molecules bind one RNA cooperatively. The lower panel shows the fitting using a cooperative dimer model. (C) Hill plot of the RNA binding data shown in B. Only the region in the binding transition is shown. (D) The molecular weight and protein–RNA ratio of the NC1–pri-mir-30a complex were estimated using SEC. The left y-axis indicates the absorption at 260, 280, and 450 nm. The right y-axis indicates the molar ratios of NC1 and the pri-mir-21 RNA estimated using either the A260/A280 ratios (shown in green) or based on the A450 values (red). (E) The SEC chromatogram of the pri-mir-21 RNA in the absence of proteins.