FIG. 2.
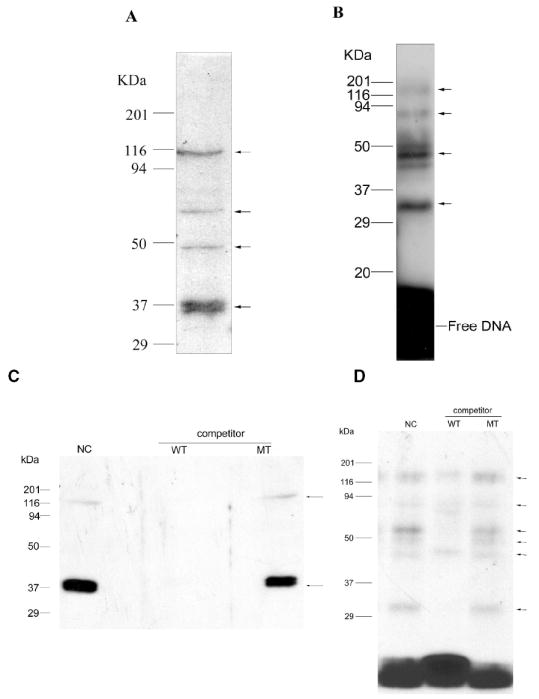
Southwestern blot and UV crosslinking of DNA-protein adducts determined the appropriate molecular masses of the proteins bound with the WT element. (A) Southwestern blot analysis. Nuclear extract (50 μg each) was separated by 15% SDS-PAGE and then transferred to nitrocellulose membrane, the proteins on the blots were denatured and renatured by serial dilution of guanidine/HCl. The membrane was probed with radiolabeled WT element (1.5 nM). The dried membrane was analyzed by autoradiography. (B) UV crosslinking of DNA-protein adducts was performed by incubating 10 μg nuclear extract with 1.6 nM radiolabeled element followed by UV crosslinking. The crosslinked complex was separated from free DNA by 15% SDS-PAGE. The molecular mass of the markers is shown on the left. Arrows indicate the bands bound with the element. (C) and (D) the same as in A and B, respectively, but showing either the effect of no competitor (NC) or the effect of adding a 100-fold excess of unlabeled wild type (WT) or mutant (MT) DNA to the radiolabeled wild type DNA used as the probe.
