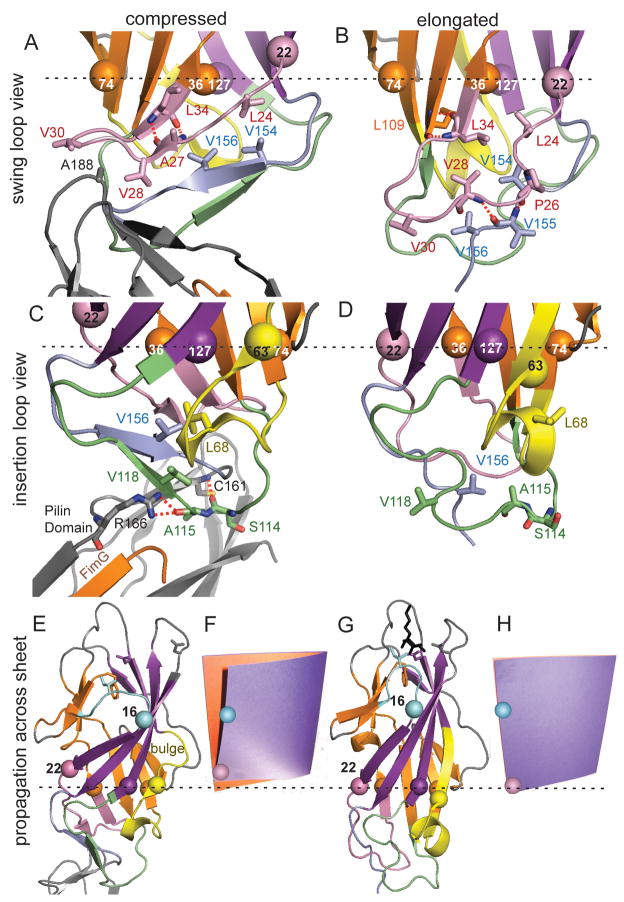
Figure 4. Structural changes caused by pilin domain interaction.
Colors and representations are the same as in Figures 1 and 3. The Cα atoms are shown as spheres for three β-sheet residues (36, 74, and 127) that are in a plane perpendicular to the field of view, as shown by the dotted black line. Residues 22 and 63 are also shown as spheres to show how they move relative to this plane and the other residues. A, B. View from side of swing loop. C, D. View from side of insertion loop. E, G. Propagation of conformational changes from the proximal region to the distal mannose-binding site. F, H. photograph of a cardboard folder with an orange page representing the split β-sheet and a purple page representing the large β-sheet in an unperturbed state (F) and with the lower left front edge pushed upward (H), to demonstrate the analogy for the page-turning mechanism of β-sandwich allostery.