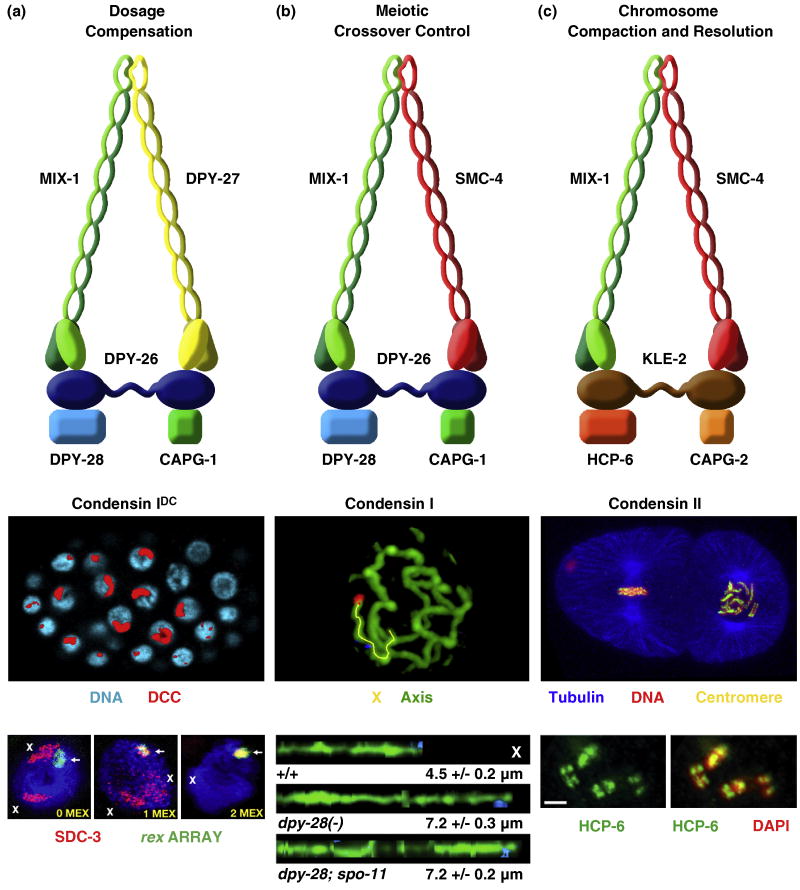
Figure 3. Three condensin complexes carry out distinct functions in C. elegans.
(a) The dosage compensation complex (the DCC, also called condensin IDC) (top) resembles condensin I. It equalizes X-linked expression between the sexes (XX hermaphrodites and XO males) by reducing transcript levels by half in hermaphrodites. Shown is an XX embryo stained with the DCC component SDC-2 (red) and DAPI (blue) (middle). The DCC binds to both X chromosomes. Shown also are images of gut cell nuclei carrying extrachromosomal arrays with multiple copies of rex-1 derivatives stained with DAPI (blue), SDC-3 antibodies (red), and array FISH (green) (bottom). Arrays have rex-1 fragments with different numbers of MEX motifs. Left, a 33 bp rex-1 fragment in which its single MEX motif was mutated, thus abrogating DCC binding. X staining is apparent (red). Middle, a wild-type 33 bp rex-1 fragment with 1 MEX motif. SDC-3 colocalizes with the array and X. Right, a 60 bp rex-1 fragment with 2 MEX motifs. SDC-3 binds robustly to rex-1 and is titrated from X, showing that MEX motifs collaborate to recruit the DCC. An array carrying a rex-1 fragment that titrates the DCC from X can suppress the male lethality caused by mutation of xol-1 in XO embryos.
(b) Condensin I. This complex differs from DCC condensin by a single subunit, SMC-4 (top). This complex controls meiotic DSB distribution through effects on chromosome structure. It also plays minor roles in chromosome segregation in mitosis and meiosis. Shown is a high resolution image of pachytene chromosomes in wild-type animals labeled with the axis protein HTP-3 and FISH probes (blue, red) to X (middle). X chromosomes from wild-type and dpy-28 mutant animals were traced (yellow) in three dimensions and straightened computationally (bottom). Straightened chromosomes are shown horizontally. Genotypes, average total axis length, and standard error of the mean are below each axis. Disruption of dpy-28 dramatically increases the X-chromosome axis length in a manner independent of DSBs made by SPO-11. In response, DSBs are increased in number and redistributed in dpy-28 mutations, causing an increase in crossovers and their redistribution.
(c) Condensin II. This complex shares one subunit (MIX-1) with the DCC (condensin IDC) and two subunits (MIX-1 and SMC-4) with condensin I (top). This complex is the prime condensin complex responsible for mitotic and meiotic chromosome compaction and resolution. Condensin II binds to the centromeres on the holocentric mitotic chromosomes. Shown is a two cell embryo (middle) with one cell in metaphase (left) and one in prometaphase (right). Centromeric proteins (yellow) and condensin II bind along the outer edge of each chromosome, adjacent to where the mitotic spindle (tubulin, blue) attaches. Condensin II also binds to meiotic chromosomes at pachytene exit to create the compact shape (diakinesis bivalents) required for chromosome segregation. Shown are the four sister chromatids of meiotic diakinesis bivalents (bottom) stained with HCP-6 antibodies (green) and DAPI (red). Merge is yellow.