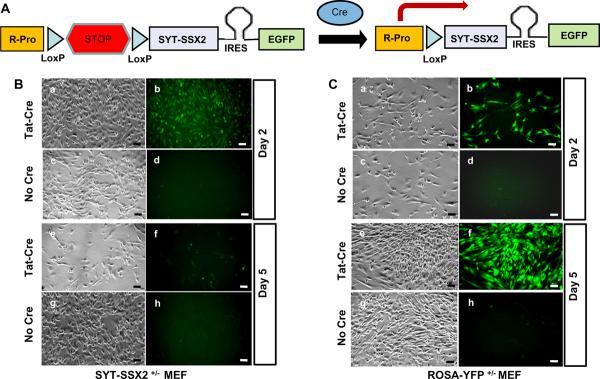
Figure 1.
Figure A shows the strategy for conditional expression of SYT-SSX2 and EGFP proteins in the genetically engineered SSM2 mice. Cre mediates recombination between the two LoxP sites in SSM2 mice thereby removing the transcriptional stop signal and allowing transcription (arrow) of the SYT-SSX2 –IRES-EGFP bicistronic mRNA from the endogenous ROSA promoter. The bicistronic mRNA is translated into distinct SYT-SSX2 and EGFP proteins via the EMCV virus derived Internal Ribosomal Entry site (IRES) placed between the coding sequences of SYT-SSX2 and EGFP.
SYT-SSX2+/− fibroblasts (B) from SSM2+/− embryos and control ROSA-YFP+/− fibroblasts (C) from ROSAY-YFP+/− embryos were cultured. SYT-SSX2+/− and ROSA-YFP+/− fibroblasts were divided into two groups; those exposed to TAT-Cre protein at 3 μm concentration for 2 hours and those not exposed to TAT-Cre. By the second day, SYT-SSX2+/− fibroblasts exposed to TAT-CRE (Ba and Bb) showed SYT-SSX2 expression based on EGFP induced fluorescence (Bb) while those not exposed to TAT-CRE (Bc and Bd) showed no fluorescence (Bd). Control ROSA-YFP+/− cells exposed to TAT-Cre (Ca and Cb) showed robust YFP expression (Cb) while those not exposed to TAT-CRE (Cc and Cd) showed no YFP expression (Cd).
Upon serially tracking the same zones, by day five SYT-SSX2+/− fibroblasts exposed to TAT-CRE (Be and Bf) showed significantly less growth (Be) and very few EGFP expressing cells (Bf) while those not exposed to TAT-CRE showed robust growth (Bg) with no EGFP expression (Bh). Control ROSA-YFP+/− cells exposed to TAT-CRE continued to show robust growth (Ce) and YFP expression (Cf) while those not exposed to TAT-CRE showed robust growth (Cg) without YFP expression (Ch). All scale bars = 50 μm.