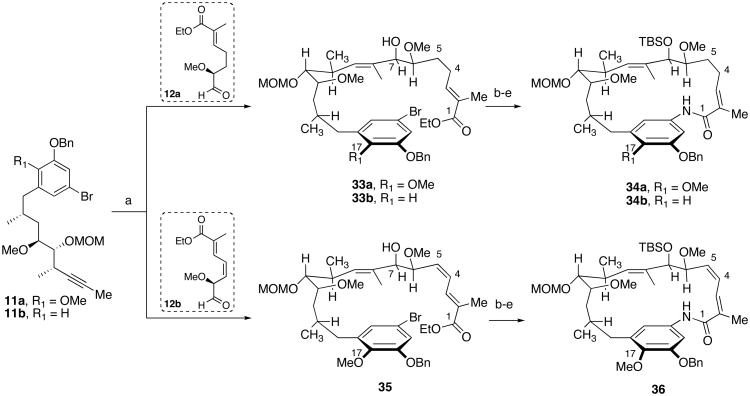
Scheme 6.
Synthesis of the Macrocyclic Cores of Phenolic Derivativesa
a Reagents and conditions: (a) (i) 2 equiv. Cp2ZrHCl, 11a or 11b, toluene, 50 °C, (ii) ZnMe2, toluene, −65 °C, (iii) 12a or 12b, 0 °C, 53–70% for 33a, 40–89% for 33b, 51–62% for 35, dr = 20:1; (b) TBSOTf, 2,6-lutidine, CH2Cl2, 0 °C; (c) LiOH, THF/MeOH/H2O; (d) (i) (CH3)2CHCH2OCOCl, NEt3, CH2Cl2, −20 °C, (ii) NH3 (l); (e) CuI, N,N′-dimethylethylenediamine, K2CO3, toluene, 100 °C, 36 h, >80% for 34a, 80% yield for 34b, 82% yield for 36.