Table 1.
Enantioselective Propargylations with Methanesulfonamide
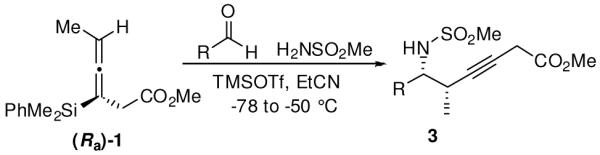 | ||||
|---|---|---|---|---|
| entry | aldehyde | yield (%)a | drb | product |
| 1 |
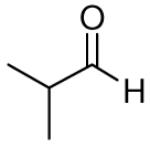
|
81 | >20:1 | 3a |
| 2 |
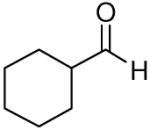
|
77 | >20:1 | 3b |
| 3 |
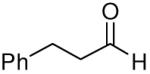
|
81 | 10:1 | 3c |
| 4 |
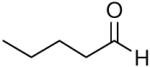
|
69 | 5:1 | 3d |
| 5 |
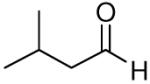
|
64 | 7:1 | 3e |
| 6 |
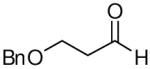
|
65 | 9:1 | 3f |
| 7 |
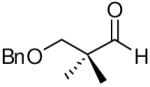
|
82c | >20:1 | 3g |
| 8 |
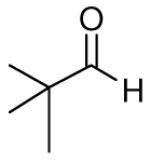
|
82c | >20:1 | 3h |
Isolated yields after purification over silica gel.
Diastereomeric ratios determined by 1H NMR analysis on crude material.
Reaction run at −45 °C in MeCN.
