FIGURE 5.
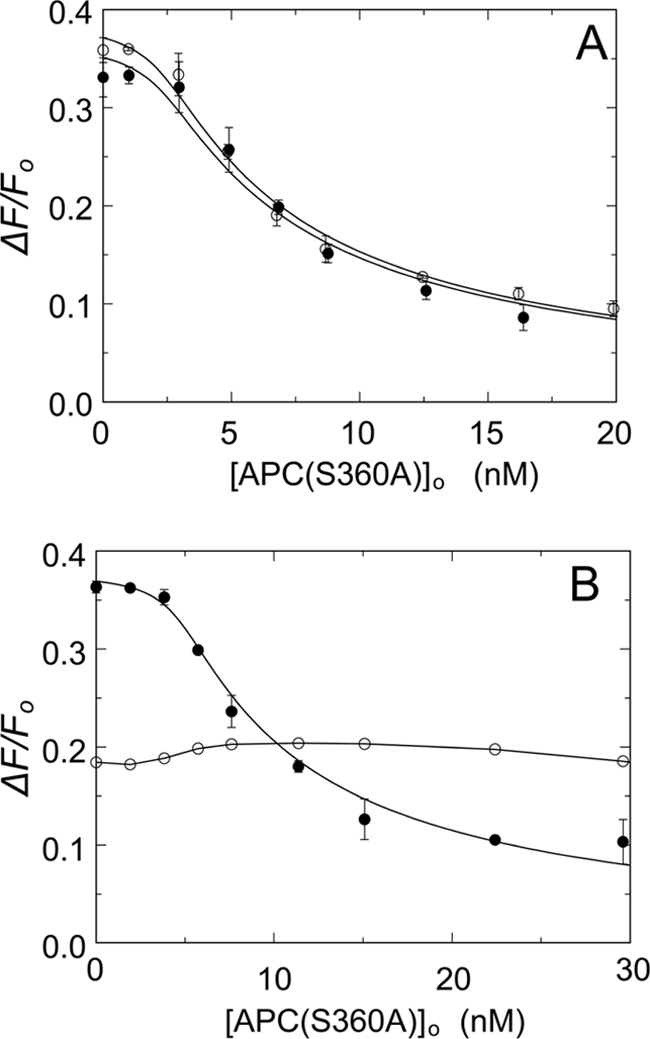
Effect of protein S or prothrombin on the binding of APC(S360A) and OG-FXa to FVa. A, titrations of mixtures of 1.2 nm OG-FXa and 3.5 nm native FVa in the presence of 50 μm phospholipid vesicles (20:60:20, DOPS:DOPC:DOPE) with APC(S360A) in the absence (●) and presence of 150 nm protein S (○). The solid lines represent fits of the data by the cubic competitive binding equation with fixed parameters of n = 0.82 ± 0.18 FVa/OG-FXa (mol/mol), KP = 0.14 ± 0.06 nm, m = 1, and fitted parameters in the absence of protein S, KC = 0.21 ± 0.05 nm and ΔFmax/Fo = 37 ± 2%, and the presence of protein S, KC = 0.21 ± 0.04 nm and ΔFmax/Fo = 39 ± 2%. B, titrations as in A of 1.2 nm OG-FXa and 5.7 nm native FVa in the absence (●) and presence of 0.6 μm prothrombin (○). The titration in the absence of prothrombin was analyzed with the fixed parameters as in A and fitted KC = 0.17 ± 0.03 nm and ΔFmax/Fo = 38 ± 1%. The error bars represent the 95% confidence interval. The solid line for the titration in the presence of prothrombin was arbitrarily drawn through the data. Fluorescence titrations were performed and analyzed as described under “Experimental Procedures.”
