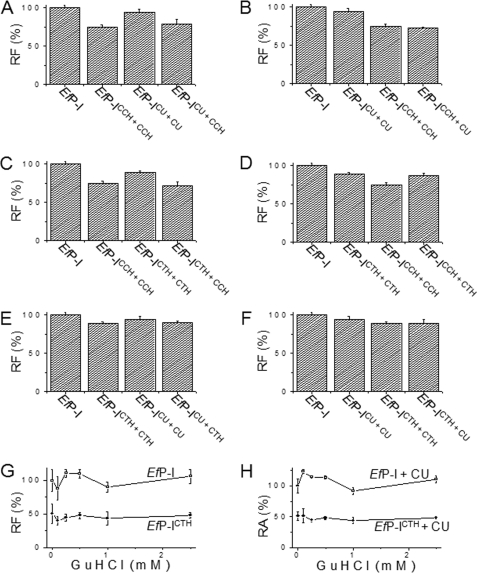
FIGURE 5.
Conformational changes of EfP-I induced repetitively by different substrates. The effects of different substrates on the conformation of the enzyme were determined by comparing the intensity of the intrinsic fluorescence. To exclude disturbance from the residual substrates and products, each sample was ultrafiltrated before fluorescence was measured. EfP-I, native protease; EfP-ICCH, enzyme reacted with CCH and ultrafiltrated to remove residual substrates and products; EfP-ICCH+CCH, enzyme reacted with CCH and ultrafiltrated and then reacted again with CCH and ultrafiltrated; EfP-ICCH+CU, enzyme reacted with CCH and ultrafiltrated and then reacted again with CU and ultrafiltrated. A, effect of CCH on the conformation of EfP-ICU. B, effect of CU on EfP-ICCH. C, effect of CCH on EfP-ICTH. D, effect of CTH on EfP-ICCH. E, effect of CTH on EfP-ICU. F, effect of CU on EfP-ICTH. EfP-I, EfP-ICCH+CCH, EfP-ICU+CU, and EfP-ICTH+CTH were used as controls. Error bars represent the S.D. G, intrinsic fluorescence of EfP-ICTH at different concentrations of guanidine hydrochloride (GuHCl), with native EfP-I used as control. H, urokinase-like activity of EfP-ICTH at different concentrations of guanidine hydrochloride. Error bars represent the S.D.