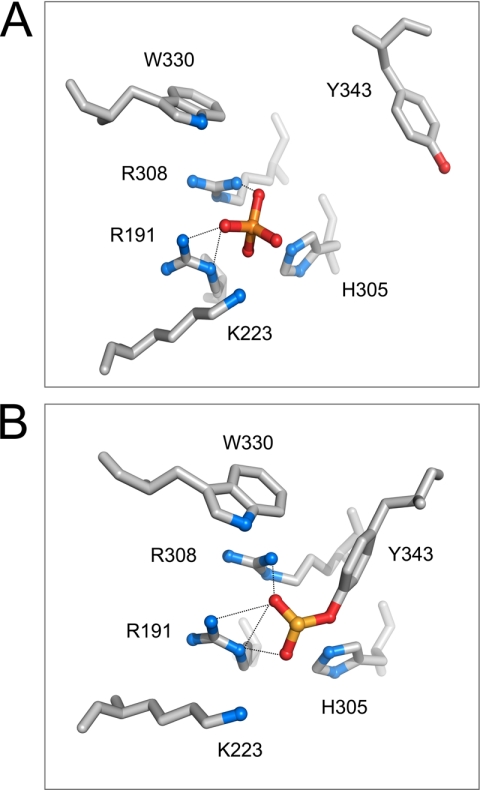
FIGURE 1.
Organization of active site residues in DNA-bound Flp with the scissile phosphate in its uncleaved and cleaved (covalently linked) states. The catalytic pentad residues (Arg-191, Lys-K223, His-305, Arg-308, and Trp-330) of a Flp monomer and the active site tyrosine (Tyr-343) donated in trans from a second monomer are displayed surrounding the scissile phosphate (gold sphere). In one active site configuration (A), the scissile phosphate is not cleaved; in the other (B), the scissile phosphate is cleaved and linked to the trans-donated Tyr-343. Note that Arg-191 and Arg-308 make hydrogen bonding interactions with the nonbridging oxygens of the scissile phosphate in both A and B (dotted lines). The structural information is from Protein Data Bank entry 1M6X (21) and is displayed using the PyMOL molecular graphics system (DeLano Scientific).