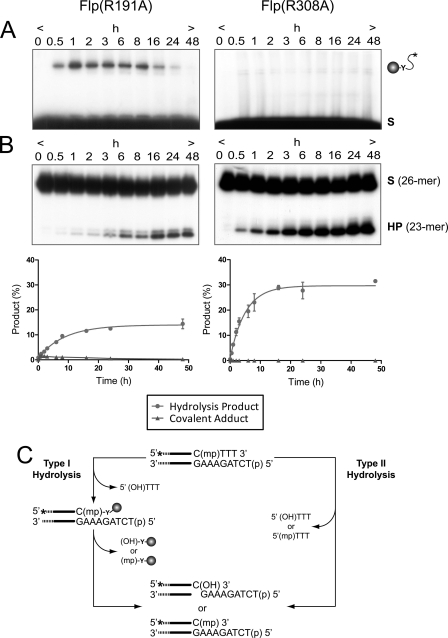
FIGURE 2.
Endonuclease activities of Flp(R191A) and Flp(R308A) on a MeP half-site substrate. A and B, the reactions were analyzed simultaneously by SDS-PAGE (A) for formation of covalent Flp-DNA adduct by Tyr-343-mediated strand cleavage and by denaturing urea-PAGE (B) for formation of the 23-mer hydrolysis product (HP). The end-labeled double-stranded DNA half-site substrate (A) or the labeled single-stranded DNA (26-mer) resulting from its denaturation (B) is denoted by (S). The 5′-OH on the bottom strand of the substrate was phosphorylated to prevent “strand joining.” The respective plots for Flp(R191A) and Flp(R308A) represent the mean values from three independent experiments with the standard errors indicated by bars. C, type I and type II endonuclease activities leading to hydrolysis of the MeP-tyrosyl intermediate and direct hydrolysis of MeP, respectively, are schematically depicted. The asterisk indicates a 32P-label at the 5′-end of the scissile top strand; (mp), (p), and (HO) represent the scissile MeP, 5′-terminal phosphate, and 5′-terminal hydroxyl, respectively. The filled circle represents Flp recombinase with its active site tyrosine (Y).