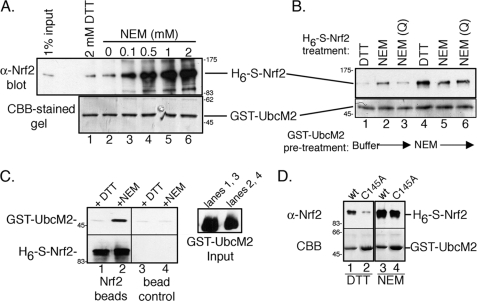
FIGURE 1.
UbcM2 and Nrf2 interact directly in vitro under conditions that promote cysteine alkylation. A, recombinant pulldown assay with GST-UbcM2 and H6-S-Nrf2 in the presence of 2 mm DTT (lane 1) or increasing amounts of NEM (lanes 2–6). The top panel shows precipitated H6-S-Nrf2 detected with α-Nrf2, and the bottom panel shows immobilized GST-UbcM2 detected with CBB. 1% of the H6-S-Nrf2 input is shown in the leftmost lane of the top panel. B, similar assay to A to determine whether Nrf2, UbcM2, or both proteins require modification by NEM to induce complex formation. GST-UbcM2 immobilized on GSH-Sepharose was either pre-treated with buffer (lanes 1–3) or NEM (lanes 4–6) for 1 h, and excess NEM was washed away. For samples in which soluble H6-S-Nrf2 was pre-treated with NEM, the excess alkylating agent was quenched with DTT (lanes 3 and 6). For all other samples, H6-S-Nrf2 was added to binding reactions supplemented with either DTT (lanes 1 and 4) or NEM (lanes 2 and 5). C, same as A except H6-S-Nrf2 was immobilized on Ni2+-nitrilotriacetic acid resin and combined with GST-UbcM2 pre-treated with DTT (lanes 1 and 3) or NEM (lanes 2 and 4). GST-UbcM2 inputs are shown in the right panel. GST-UbcM2 was detected with anti-GST and H6-S-Nrf2 with α-Nrf2. D, same as A testing the requirement for an intact active-site cysteine (Cys-145) in UbcM2. All experiments were repeated three independent times.