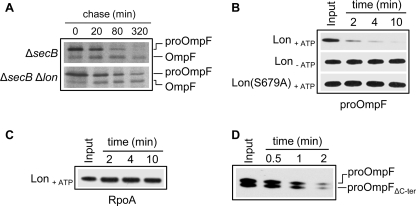
FIGURE 4.
ProOmpF is efficiently degraded by Lon both in vivo and in vitro. A, pulse-chase analysis shows the processing of 35S-labeled proOmpF expressed in SG20781 ΔsecB or ΔsecB Δlon isogenic mutants harboring plasmid p29-OmpF, grown and labeled at 30 °C, and chased for various times after incubation at 16 °C. B, in vitro degradation of urea-denatured proOmpF (0.3 μm) by Lon6 (0.16 μm) in the presence of ATP and in the absence of ATP (Lon-ATP) or by Lon6 (S679A) (0.16 μm) in the presence of ATP. C, shown are in vitro degradation assays of urea-denatured RpoA (0.3 μm) in the presence of Lon6 (0.16 μm) and ATP under the same conditions as those for proOmpF in B. D, degradation of proOmpF and proOmpFΔC-ter missing the last 10 amino acids (353TVAVGIVYQF362) by Lon6 (0.16 μm) is shown. For the assay both proOmpF (0.15 μm) and proOmpF ΔC-ter (0.15 μm) were added to the same reaction mix.