Table 1.
Redox transitions and rate constants of the fast kinetics of reduction by NADH of wild-type Na+-NQR, NqrB-D346A and NqrB-D397A mutants
| Rate constants (s-1) |
|||||
| Enzyme | NaCl | K1 | K2 | K3 | K4 |
| WT | |||||
| 0 | 249.3 | 15.4 | 4.2 | 0.31 | |
| FAD → FADH2 | RibH• → RibH2 | 2(FMN → FMN•-) | 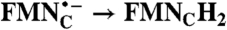 |
||
| 100 | 143.2 | 35.1 | 0.7 | ||
| FAD → FADH2; RibH• → RibH2 | 2(FMN → FMN•-) | 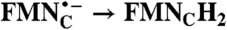 |
|||
| NqrB-D346A | |||||
| 0 | 235 | 20.1 | 3.6 | 0.3 | |
| FAD → FADH2 | 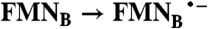 |
 ; Rib → RibH• ; Rib → RibH•
|
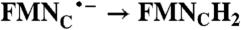 |
||
| 50 | 121.6 | 16.4 | 1.5 | 0.2 | |
FAD → FADH2; 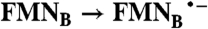
|
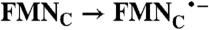 ; Rib → RibH• ; Rib → RibH•
|
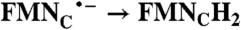 |
RibH• → RibH2 | ||
| NqrB-D397A | |||||
| 0 | 268 | 4.2 | 0.3 | ||
| FAD → FADH2 | RibH• → RibH2; 2(FMN → FMN•-) | 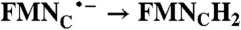 |
|||
| 50 | 270 | 4.6 | 0.35 | ||
| FAD → FADH2 | RibH• → RibH2; 2(FMN → FMN•-) | 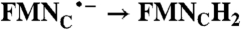 |
|||
| 200 | 258 | 5.1 | 0.4 | ||
| FAD → FADH2 | RibH• → RibH2; 2(FMN → FMN•-) | 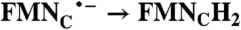 |
|||
Desalted samples were mixed with 250 μM NADH using different concentrations of sodium.
