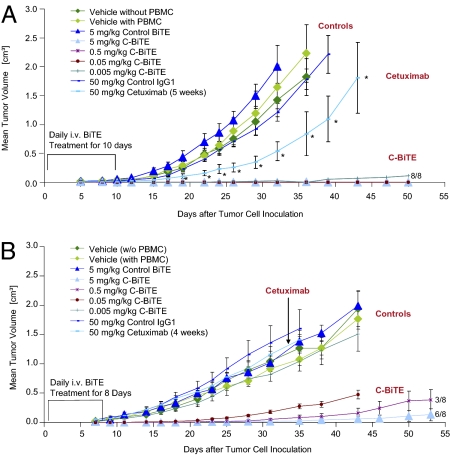
Fig. 3.
Antitumor activity of C-BiTE antibody and parental cetuximab in two human CRC xenograft mouse models Tumor cell doses were determined upfront, allowing a rapid and reproducible outgrowth of tumors from HCT116 (A) or HT29 human CRC cell lines (B) in NOD/SCID mice. HT-29 (2× 106 cells per inoculum) or human colon carcinoma cell line HCT116 (5× 106 per inoculum) were injected s.c. together with PBMCs from healthy human donors at an E:T cell ratio of 1:2 in the right dorsal flank of female NOD/SCID mice (n = 8 per group). As indicated, four different control conditions were tested for their influence on tumor outgrowth: vehicle in the absence, and vehicle, isotype IgG1, and a control BiTE antibody binding to CD3 and an irrelevant hapten antigen in the presence of human PBMCs. Cetuximab was intraperitoneally administered twice a week starting on d 1 at 50 mg/kg for 4 (HT29) or 5 wk (HCT116), whereas C-BiTE was daily administered by i.v. bolus starting on d 1 only for the first 8 d (HT29) or 10 d (HCT116). Tumor growth was determined by external caliper measurements, and tumor volumes were calculated using a standard hemiellipsoid formula (Eq. 2). Values represent mean tumor size (in cm3) ± SEM (n = 8 per group). Growth retardation by cetuximab in (A) was statistically significant for all time points labeled by an asterisk (P < 0.05). For HCT116 xenografts, all BiTE responses were highly significant, and at all four doses tested, all eight mice did not develop tumors. For HT29, all but the lowest dose of 0.005 mg/kg resulted in a statistically significant inhibition of tumor growth (Student t test).