Figure 3.
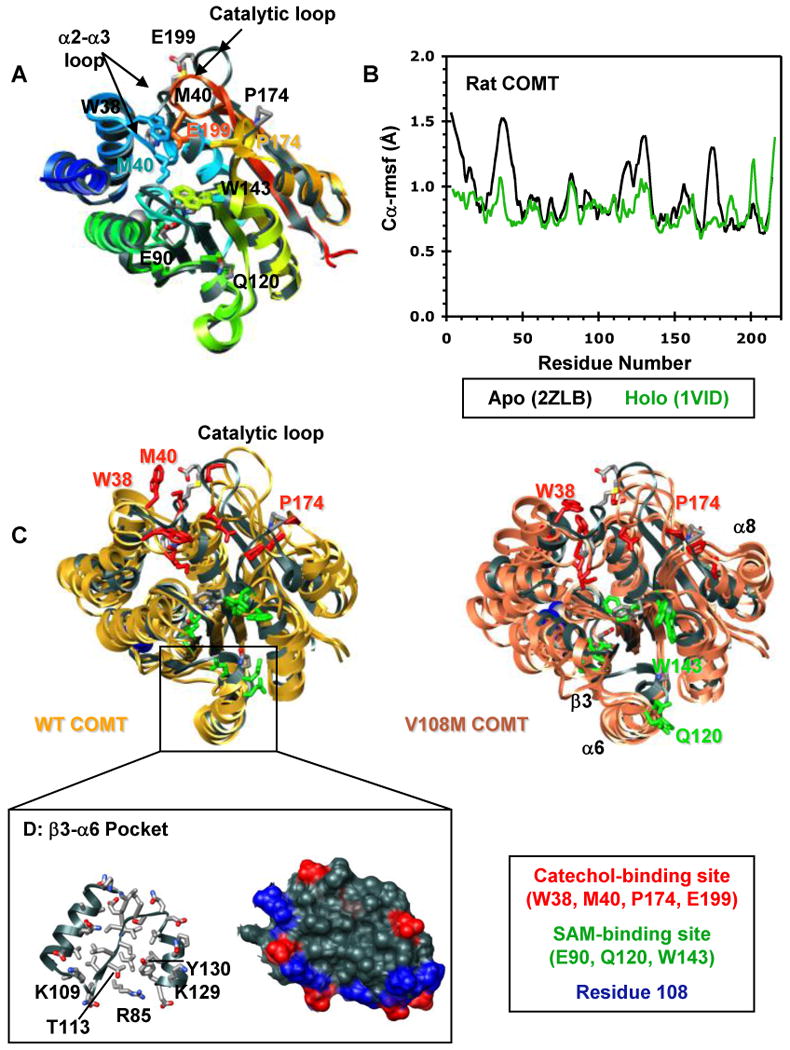
Structural characterization of the COMT apoprotein. (A) Superposed ribbon diagrams of the rat COMT apoprotein (gray, 2ZLB.PDB (66)) and the rat (1VID.PDB (53)) and human (3BWM.PDB (54)) holoproteins bound with SAM and DNC and colored from blue (N-terminus) to red (C-terminus). The overall structures of the rat and human holoproteins are virtually identical. The rat apo and holoproteins differ mainly in the loop regions that define the catechol-binding site. The α2-α3 loop, β5-α8 loop, and the catalytic loop (residues 197-202) pull away from the protein core in the apoprotein exposing the SAM- and catechol-binding sites. This open configuration may facilitate substrate binding. Interestingly, the adenosine pocket of the apo and holoprotein SAM-binding sites are very similar showing only a slight displacement of key active site residues (E90, Q120, W143). Active site residues are shown in stick representation and colored/labeled in either gray (apoprotein) or to match the structure (holoprotein). (B) Cα-rmsf values (Å) per residue for the rat apoprotein (black, 2ZLB.PDB (66)) and holoprotein (green, 1VID.PDB (53)). (C) Structural overlay of the rat apoprotein crystal structure (gray, 2ZLB.PDB (66)) with structures from the final ns of three independent simulations of human WT (gold) and V108M (pink) COMT. The human WT and rat apoproteins behave similarly in that the structure of the adenosine pocket in the SAM-binding site (α5, α6, α7) is maintained, with some flexibility in the orientations of active site residues E90, Q120, and W143, while the α2-α3, β5-α8, and catalytic loops fluctuate greatly opening up the active-site core and moving W38, M40, P174 and E199 out of the substrate-binding pocket. In addition to these motions, both β3 and α6 pull away from the protein core during simulations of V108M COMT. SAM- and catechol-binding residues are shown in stick representation and colored in green and red, respectively. Residue 108 is shown in space-filling representation and colored blue. (D) Ribbon (left) and van der Waals surface (surface) representation of the β3-α6 pocket from human WT COMT. The V108M polymorphism alters the orientations of β3 and α6, possibly destabilizing the protein. Therefore, the β3-α6 pocket may serve as a target site for docking small molecules to stabilize the V108M COMT structure. Positively and negatively charged residues in the surface representation are colored in blue and red.
