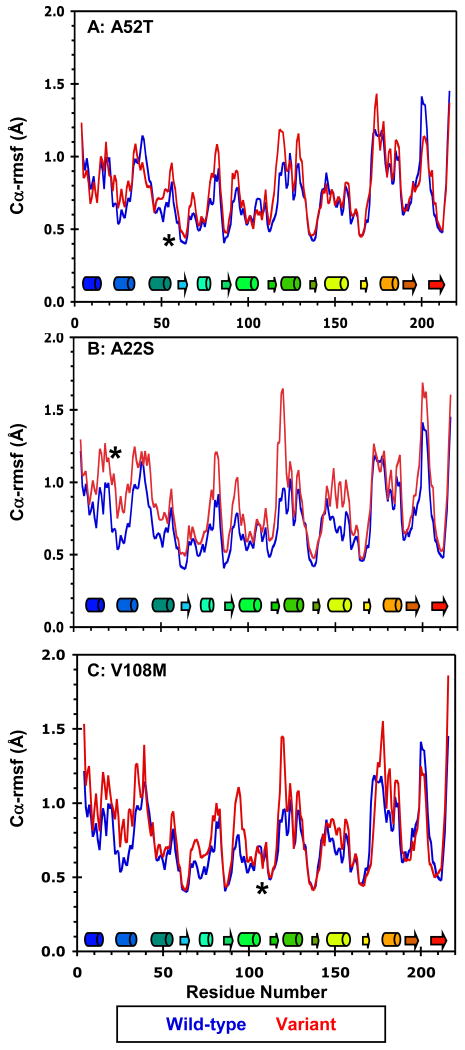
Figure 4.
Mobility of the wild-type, A22S, A52T, and V108M COMT variants at 37°C. Cα-rmsf values (Å) per residue for the wild-type protein (blue) compared with those of the (A) A52T, (B) A22S, and (C) V108M COMT variants (all in red). Cα-rmsf values were calculated relative to the average structure over the last 10 ns of each simulation. Secondary structural elements are depicted as cylinders for α-helices and arrows for β-strands and are colored to match the structure in Figure 1. The polymorphic residues are marked by asterisks.