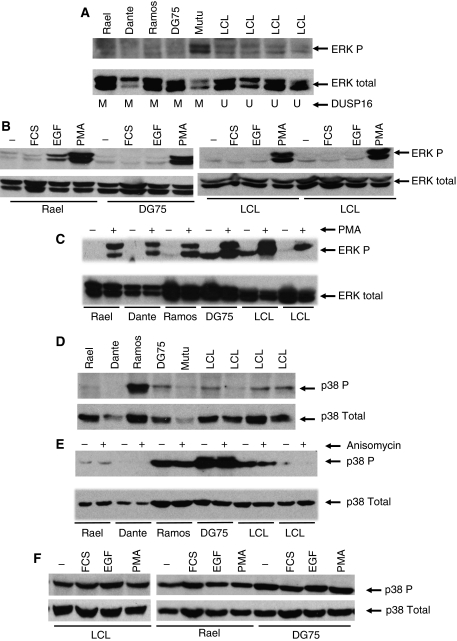
Figure 3.
ERK and p38 activity is independent of the methylation status and expression of DUSP16 in BL. (A) Constitutive ERK activity is not elevated in BL relative to LCLs and is unrelated to the expression and methylation status of DUSP16. Protein lysates were prepared from exponentially growing BL cell lines and LCL as indicated and ERK activity determined by western blotting as described in Materials and Methods section. (B) ERK phosphorylation is induced by PMA but not serum or EGF in BL and LCL. The indicated BL cell lines were serum-starved overnight then challenged with 40% serum (FCS), EGF or phorbol ester (PMA) as indicated and both total ERK and phosphorylated ERK levels were determined by western blotting as described in Materials and Methods section. Only phorbol ester reproducibly and robustly induces ERK activity under these conditions. (C) Inducible ERK phosphorylation is not influenced by the methylation status of DUSP16. The indicated cell lines were serum-starved overnight then treated with PMA as indicated and both total ERK and phosphorylated ERK determined by western blotting as described in Materials and Methods section. (D) Constitutive p38 activity is not elevated in BL relative to LCL and is unrelated to the expression and methylation status of DUSP16. Protein lysates were prepared from exponentially growing BL cell lines and LCLs as indicated and total and phosphorylated p38 activity was determined by western blotting as described in Methods. (E) Anisomycin does not activate p38 in BL or LCLs. The indicated cell lines were exposed to anisomycin and total and phosphorylated p38 levels were determined by western blotting as described in Materials and Methods section. (F) Activation of p38 by mitogens is not related to expression or methylation of DUSP16. The indicated BL cell lines and LCLs were serum-starved overnight then challenged with 40% serum (FCS), EGF or phorbol ester (PMA) as indicated and both total p38 and phosphorylated p38 levels were determined by western blotting as described in Materials and Methods section.