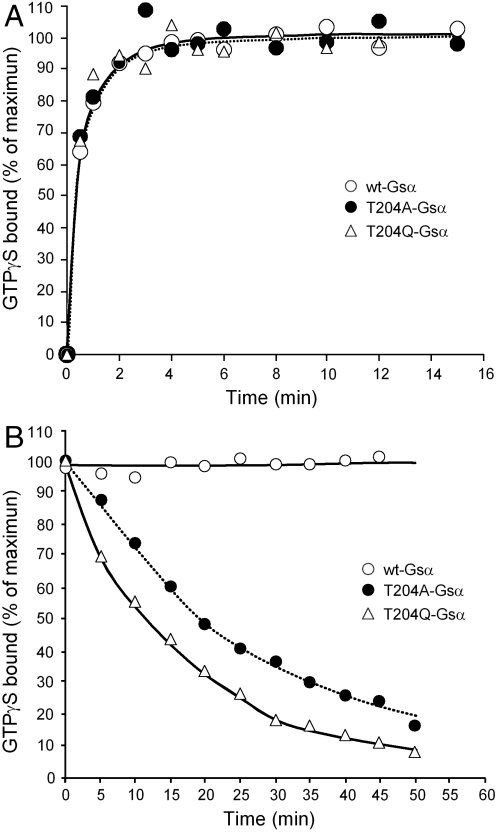
Fig. 4.
Increased rates of dissociation of GTPγS from T204A-Gsα and T204Q-Gsα. (A) Association of GTPγS to wild-type Gsα, T204AGsα, and T204Q-Gsα. Five pmol of GTPγS binding sites were incubated at 32 °C with 1,000 nM [35S]GTPγS (specific activity 16,000 cpm/pmol). At the indicated times, aliquots were removed and the bound [35S]GTPγS was determined as is described in Materials and Methods. (B) Dissociation of GTPγS from wild-type and mutant Gsα. Wild-type and mutants Gsα were first incubated as in A with 1,000 nM [35S]GTPγS for 15 min at 32 °C. At time 15 min (time zero on the graph) unlabeled GTPγS was added to a final concentration of 1 mM, and 100-μL samples were collected at the indicated times in order to analyze the [35S]GTPγS remaining in a protein-bound state.