Abstract
TNF, acting through p55 tumor necrosis factor receptor 1 (TNFR1), contributes to the pathogenesis of many inflammatory diseases. TNFR-associated periodic syndrome (TRAPS, OMIM 142680) is an autosomal dominant autoinflammatory disorder characterized by prolonged attacks of fevers, peritonitis, and soft tissue inflammation. TRAPS is caused by missense mutations in the extracellular domain of TNFR1 that affect receptor folding and trafficking. These mutations lead to loss of normal function rather than gain of function, and thus the pathogenesis of TRAPS is an enigma. Here we show that mutant TNFR1 accumulates intracellularly in peripheral blood mononuclear cells of TRAPS patients and in multiple cell types from two independent lines of knockin mice harboring TRAPS-associated TNFR1 mutations. Mutant TNFR1 did not function as a surface receptor for TNF but rather enhanced activation of MAPKs and secretion of proinflammatory cytokines upon stimulation with LPS. Enhanced inflammation depended on autocrine TNF secretion and WT TNFR1 in mouse and human myeloid cells but not in fibroblasts. Heterozygous TNFR1-mutant mice were hypersensitive to LPS-induced septic shock, whereas homozygous TNFR1-mutant mice resembled TNFR1-deficient mice and were resistant to septic shock. Thus WT and mutant TNFR1 act in concert from distinct cellular locations to potentiate inflammation in TRAPS. These findings establish a mechanism of pathogenesis in autosomal dominant diseases where full expression of the disease phenotype depends on functional cooperation between WT and mutant proteins and also may explain partial responses of TRAPS patients to TNF blockade.
Keywords: autoinflammatory disease, genetic disease
TNF-receptor type 1 (TNFR1, TNFRSF1A, TNFR p55), a ubiquitously expressed surface receptor in the TNFR superfamily, is mainly responsible for the inflammatory effects of TNF in mouse and man. TNFR1 mediates an intracellular signaling cascade through its intracellular death domain and the TNFR-associated death domain adaptor (TRADD) protein, rapidly triggering activation of NF-κB and MAPK. Both of these signaling pathways independently induce gene expression of inflammatory cytokines and chemokines. Additionally, genes induced by NF-κB block secondary proapoptotic signaling complexes, resulting in a proinflammatory effect of TNFR1 in most cell types (1, 2).
Missense mutations in TNFRSF1A, the gene encoding TNFR1, are the cause of the autosomal dominant autoinflammatory disease, TNFR-associated periodic syndrome (TRAPS) (3, 4). This syndrome is characterized by recurrent prolonged episodes of fever and inflammation, which occur either spontaneously or after minor triggers. Symptoms include fever, serositis, migratory rashes, and myalgia associated with inflammatory fasciitis. TRAPS also can be complicated by systemic amyloidosis (4). TRAPS is part of an emerging group of diseases termed “autoinflammatory” that involve abnormal activation of the innate immune system in the absence of autoantibodies or autoreactive T cells. Other autoinflammatory diseases include the cryopyrinopathies (or cryopyrin-associated periodic syndromes) linked to dominant active mutations in NLRP3/cryopyrin, a component of the IL-1β–processing inflammasome. More common diseases, such as gout, systemic-onset juvenile idiopathic arthritis, and adult-onset Still's disease, also have been classified as autoinflammatory (5–7) More than 50 TNFRSF1A mutations associated with TRAPS have been described in the INFEVERS database (http://fmf.igh.cnrs.fr/ISSAID/infevers) (8). They are restricted to the extracellular domain of the receptor, with a striking absence of mutations that would result in loss of protein expression or truncation. Mutations disproportionately affect cysteine residues critical for folding of the extracellular portion of the receptor (4). Another class of mutations, exemplified by R92Q and P46L missense mutations, occurs in 1–5% of the general population (and for P46L, in up to 10% of a West African population) (9, 10). These two variants are more likely to be functional polymorphisms thought to synergize with other abnormalities to cause a milder form of TRAPS with low penetrance (11).
Originally, TRAPS mutations were thought to cause inflammation by inhibiting metalloprotease-dependent cleavage of TNFR1 that produces soluble “shed” receptors. However, some TRAPS patients have normal receptor shedding in assays of phorbol 12-myristate 13-acetate treated peripheral blood mononuclear cells (PBMC) (12). Treatment with TNF-blocking agents should cause a dramatic decrease in symptoms if decreased shedding of TNFR1 were the sole abnormality in TRAPS. However, TNF blockade does not always induce complete remission or normalization of acute-phase reactants in TRAPS (4, 13–15). All TRAPS-associated mutations in TNFR1 studied to date that are not found in the general population profoundly disrupt receptor trafficking, resulting in intracellular retention of mutant receptors in the endoplasmic reticulum (ER) (16, 17), likely because of abnormal oligomerization of mutant receptors through nonphysiological disulfide bonds. Molecular modeling also predicts misfolding of TRAPS-associated mutant receptors (18). Mutant receptors failed to interact physically with WT receptors through the physiological N-terminal preligand assembly domain and failed to bind TNF (16). Mutant receptors were not shed and did not inhibit normal trafficking or shedding of WT TNFR1. Because of these abnormalities, we refer to these mutants as “structural mutations.” In contrast, the R92Q rare polymorphic variant of TNFR1 behaved like the WT TNFR1 in terms of receptor trafficking and TNF binding, and we refer to this and other rare variants as “nonstructural” (16, 19). TRAPS patients with structural TNFR1 mutations also generally present with more severe symptoms and greater likelihood of progression to amyloidosis. These results suggested that structural mutations lead to loss of function rather than gain of function of TNFR1. How these mutations lead to a phenotype of excessive inflammation is not known.
Results
TRAPS-Associated Mutant TNFR1 Protein Accumulates Intracellularly and Does Not Function as a Conventional TNF Receptor.
To unravel the pathogenesis of TRAPS, we generated mice in which the T50M or C33Y TRAPS-associated mutations were engineered into the endogenous TNFRSF1A locus (Fig. S1). These mutant receptors fail to traffic to the cell surface in homozygous mutant mouse neutrophils as shown by FACS (16). However, Western blotting revealed an increase in total cellular TNFR1 with increasing gene dosage of the mutant allele (Fig. 1A). In general the C33Y mutation resulted in higher levels of TNFR1 than the T50M mutation (Fig. 1A). This finding is consistent with results from transfected receptors, in which cysteine mutations had the most severe trafficking defects (16). To determine whether accumulation of TNFR1 mutants was caused by decreased degradation or increased synthesis, we treated cells with cycloheximide to block new protein synthesis and measured levels of TNFR1 over time. Under these conditions, mutant TNFR1 protein was detectable for up to 8 h, whereas WT TNFR1 protein disappeared within 2 h (Fig. 1B). These data indicate that the increases in the steady-state level of TRAPS-associated mutant TNFR1 probably result from the reduced turnover of the mutant TNFR1 protein.
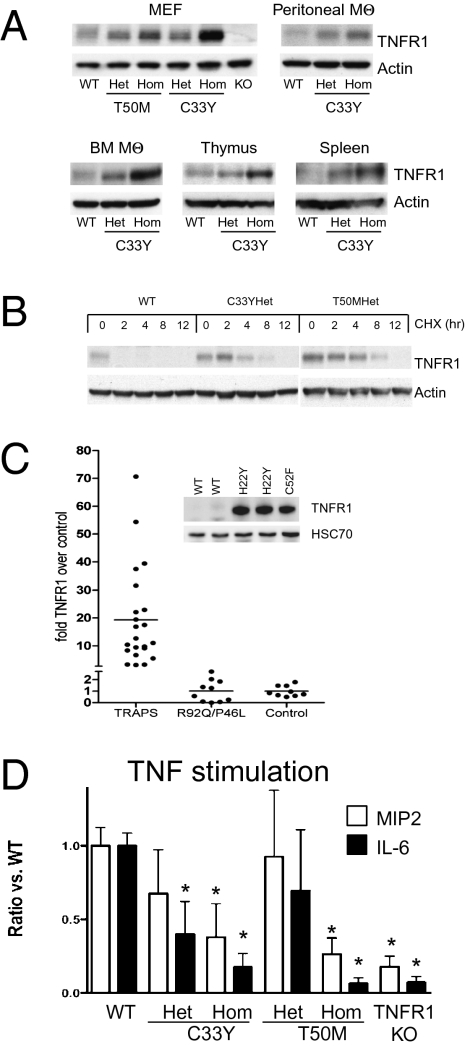
Fig. 1.
Intracellular accumulation of TNFR1 protein in TRAPS. (A) Western blots of TNFR1 in cell lysates from the indicated cell types or organs from TRAPS-knockin mice harboring the indicated heterozygous (Het) or homozygous (Hom) TNFR1 mutations. WT C57BL/6 or TNFR1 knockout (KO) mice were used as controls. (B) Increased stability of TNFR1 protein in TRAPS MEF. Cells were treated with 10 μg/mL of cycloheximide for the indicated times, and TNFR1 levels were analyzed by Western blot. (C) Quantitation of Western blots of intracellular TNFR1 in PBMC from TRAPS patients with structural TNFR1 mutations (n = 23), from patients with R92Q or P46L functional polymorphisms (n = 10), and from healthy donor controls (WT; n = 9). Fold increase was calculated by dividing the density of TNFR1 bands from the patient relative to the average of control samples run on the same gel, after correction for loading with actin or HSC70. TNFR1 levels were significantly higher in TRAPS patients than in controls (P = 0.005, by unpaired two-tail Student's t test), whereas TNFR1 levels from patients with functional polymorphisms were not significantly different from controls. Inset shows an example of the primary Western blot data for three patients heterozygous for the indicated TNFR1 mutations and two healthy donor controls. (D) Resident peritoneal macrophages from mice of the indicated genotype were stimulated with 100 ng/mL murine TNF. IL-6, and MIP-2 were measured after 6 h. The data are averages ± SEM of at least three independent experiments, each with values normalized to the secretion of cytokines by WT cells in each experiment. IL-6 and MIP-2 levels produced by WT macrophages ranged from 933–3,367 pg/mL and 5,957–47,519 pg/mL, respectively. *, comparisons with WT cells with P < 0.05 by unpaired Student's t test.
To determine whether these observations apply to patients with TRAPS, we measured total cellular levels of TNFR1 by Western blotting and found an average of 20-fold higher levels of TNFR1 in patients heterozygous for structural TRAPS mutations. By contrast, TNFR1 was expressed at normal levels in samples from patients heterozygous for the R92Q or P46L nonstructural TNFR1 variants (Fig. 1C). Although we could not detect mutant TNFR1 on the cell surface by flow cytometry (16), low levels of functional receptor still may remain. To test this possibility, we added TNF to peritoneal macrophages from heterozygous or homozygous TNFR1-mutant knockin mice and measured production of IL-6 and macrophage-inflammatory protein 2 (MIP-2), which are strongly induced by TNF in WT cells in a TNFR1-dependent manner (Fig. 1D). Production of MIP-2 and IL-6 in response to TNF was reduced in both homozygous T50M and C33Y TNFR1-mutant macrophages nearly to the level in TNFR1-deficient mac-rophages. Therefore, despite accumulation of TNFR1 intracellularly, these two TRAPS-associated mutant receptors appeared to have little or no ability to function as surface receptors for TNF. In TNFR1 heterozygous macrophages, responses to TNF were mildly reduced, with only the reduction in IL-6 production in C33Y mutants reaching statistical significance. This finding suggests that in TNFR1 heterozygous mutant macrophages, which model the genetic state of TRAPS patients, haploinsufficiency of WT TNFR1 results in only a small decrease in TNF responsiveness.
Intracellular accumulation of misfolded mutant TNFR1 in the ER might be hypothesized to induce inflammation through the unfolded protein response (UPR), which in some circumstances can induce production of proinflammatory cytokines (20). However, we did not find any spontaneous increase in the expression of the ER stress-inducible genes BiP or CHOP, and heterozygous TNFR1-mutant cells responded normally to UPR induced by the protein folding inhibitor thapsigargin (Fig. S2). Similar results were observed in monocyte-derived macrophages from TRAPS patients (Fig. S2). Thus, despite intracellular accumulation, the mutant TNFR1 in TRAPS does not appear to activate or alter the classical UPR.
Enhanced Responsiveness to LPS Dependent on JNK and p38 Kinase Activity in Cells from TNFR1-Mutant Mice.
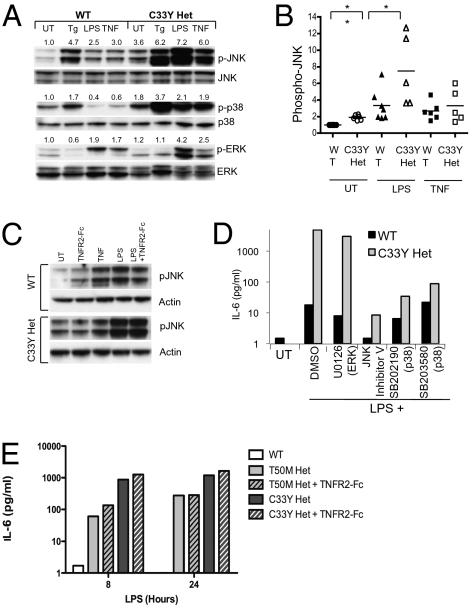
Because the death domain of all TRAPS-associated TNFR1 mutants is intact, we reasoned that, rather than triggering ER stress, intracellular accumulation of mutant TNFR1 instead may activate intracellular signaling in a TNF-independent manner. We measured baseline and induced activation of NF-κB and MAPK, two key proinflammatory signaling pathways activated by TNFR1, in mouse embryonic fibroblasts (MEF) derived from TNFR1-mutant mice. In MEF heterozygous for the C33Y TNFR1 mutation, we found significantly elevated baseline levels of JNK and p38 MAPK phosphorylation at amino acid residues known to correlate with kinase activation, whereas levels of phosphorylated ERK were near normal (Fig. 2A). The ER stress inducer thapsigargin and TNF activated JNK and p38 kinases similarly in WT and C33Y heterozygous MEF relative to the baseline in each cell type (Fig. 2A). However, LPS treatment resulted in enhanced JNK and ERK phosphorylation in TNFR1-mutant MEF above the already elevated baseline (Fig. 2A). Quantitation of results from five independent experiments confirmed significant increases in both baseline and LPS-stimulated phosphorylated JNK (pJNK) in C33Y TNFR1 heterozygous MEF compared with similarly treated WT cells, whereas JNK activation after TNF stimulation was similar in WT and TNFR1 mutant cells. (Fig. 2B). Enhanced JNK activation appeared to be independent of TNF–TNFR1 interactions, because TNF could not be detected in the supernatant of LPS-stimulated MEF, and TNFR2-Fc failed to block either the enhanced baseline or LPS-induced phosphorylation of JNK in C33Y TRAPS heterozygous MEFs (Fig. 2C). IL-6 production in response to LPS was greatly enhanced in TNFR1 heterozygous mutant MEF, and this increase could be blocked with inhibitors of either JNK and p38 kinases but not TNFR2-Fc, suggesting that mutant TNFR1 enhances LPS-induced IL-6 production in a manner dependent on these MAPKs (Fig. 2 D and E).
Fig. 2.
Enhanced IL-6 production dependent on JNK and p38 kinases in TNFR1-mutant MEF. (A) WT and C33Y heterozygous TNFR1-mutant MEF were left untreated (UT) or were treated with thapsigargin (Tg; 10 μM), LPS (100 ng/mL), or murine TNF (100 ng/mL) for 1 h. Total cell extracts were prepared and subjected to Western blot analysis to detect the levels of the indicated proteins. Numbers shown are the density of each phosphoprotein relative to nonphosphorylated protein normalized to the WT untreated sample. (B) Quantitation of the data in A for pJNK from at least five independent experiments with LPS and TNF. pJNK was calculated from the ratio of density of pJNK/JNK in each sample, and the average of untreated WT MEF was normalized to 1 for each experiment. **, P = 0.0024; *, P = 0.038 for the indicated comparisons (Student's unpaired t test). (C) JNK hyperphosphorylation is independent of TNF in TNFR1-mutant MEF. Cells of the indicated genotype were treated with the indicated agents as in A with or without 10 μg/mL TNFR2-Fc (etanercept) previously added to the cultures. (D) WT and C33Y TNFR1 heterozygous mutant MEF were pretreated for 30 min with DMSO or with 5 μM ERK inhibitor U0126, 10 μM JNK II inhibitor, 10 μM p38 inhibitor SB202190, or 10 μM p38 inhibitor SB203580 and then were stimulated with 100 ng/mL LPS for 6 h before collection of supernatants for IL-6 measurement. MTT assays confirmed lack of toxicity of these inhibitors under identical conditions. (E) WT and T50M and C33Y TNFR1 heterozygous mutant MEF were plated at 5 × 105 cells/well and left untreated or incubated with 100 ng/mL LPS for 8 h with or without 10 μg/mL TNFR2-Fc. IL-6 in supernatants was measured by cytokine bead assay. Data are averages ± SEM of three to five independent experiments.
In contrast to MAPKs, basal and LPS-stimulated NF-κB activity was either normal or slightly decreased in T50M and C33Y heterozygous TNFR1-mutant MEF (Fig. S3 A and B). Although sustained activation of JNK has been associated with enhanced cell death, particularly in the setting of impaired NF-κB activation (21), neither TNF nor LPS treatment affected viability of TNFR1-mutant cells. As previously reported (22), TNF treatment readily induced cell death in c-RelA–deficient MEF (Fig. S3C). Interferon regulatory factor 3 (IRF3) activation also was normal in cells from C33Y and T50M TNFR1 heterozygous mice treated with LPS (Fig. S3D), and induction of Regulated upon Activation, Normal T-cell Expressed and Secreted (RANTES) mRNA and protein, which is dependent on IRF3, also was relatively normal in TNFR1-mutant cells.
To determine whether mutant TNFR1 influenced TLR4 signaling in primary immune cells, we treated splenocytes with LPS and examined phosphorylation of JNK, which was the most strongly induced MAPK in these cells. In WT splenocytes, peak levels of pJNK were achieved in 15–30 min and then declined to baseline by 60 min (Fig. S4A). By contrast, LPS stimulation of heterozygous T50M TNFR1-mutant splenocytes progressively activated JNK over 60 min with no return to baseline, whereas the response in homozygous TNFR1-mutant cells was more similar to that of WT cells. In accordance with MAPK hyperactivation, heterozygous TNFR1-mutant resident peritoneal macrophages secreted more IL-1β, IL-6, TNF, and MIP-2 than did WT controls, with statistical significance observed for IL-6 (Fig. S4B). However, enhancement of IL-6 and IL1β production was reduced in homozygous TNFR1 macrophages, suggesting that signaling through the WT TNFR1 is required for the enhanced responsiveness of macrophages heterozygous for TNFR1 mutations. TNFR1-deficient macrophages did not produce less of these cytokines than WT cells, indicating that autocrine TNF–TNFR1 interactions are not required for normal acute inflammatory responses to LPS in WT cells. Taken together, these data suggest that TRAPS-associated TNFR1 mutations can enhance responses to LPS in fibroblasts without a contribution of the WT allele, whereas in macrophages, autocrine TNF acting through the WT TNFR1 receptor is required for maximally enhanced responsiveness to LPS.
Enhanced Lethality of LPS in Heterozygous TRAPS-Mutant TNFR1 Knockin Mice.
Heterozygous and homozygous T50M and C33Y TNFR1-mutant mice exhibited normal growth, development, appearance, and life span in multiple specific-pathogen–free (SPF) facilities. Consistent with a lack of surface expression of the mutant TNFR1 (16), spleens from homozygous knockin mice resembled those from TNFR1-deficient mice in lacking an intact follicular dendritic cell network and defined B- and T-cell zones (Fig. 3A). Multiplexed measurement of 23 serum cytokines in TNFR1-mutant mice failed to show any spontaneous elevation in serum cytokine concentrations, including TNF, IL-1β, and IL-6 (Fig. S5). Continuous monitoring of body temperature in a cohort of TRAPS-associated heterozygous or homozygous mutant mice for periods of up to 28 days did not reveal any alterations from normal diurnal temperature variations (Fig. S6). Thus, at least under SPF conditions, the perturbations in cell signaling induced by TNFR1 mutations homologous to those in TRAPS are not sufficient to induce spontaneous fevers or other obvious inflammatory manifestations in these two lines of TNFR1-knockin mice.
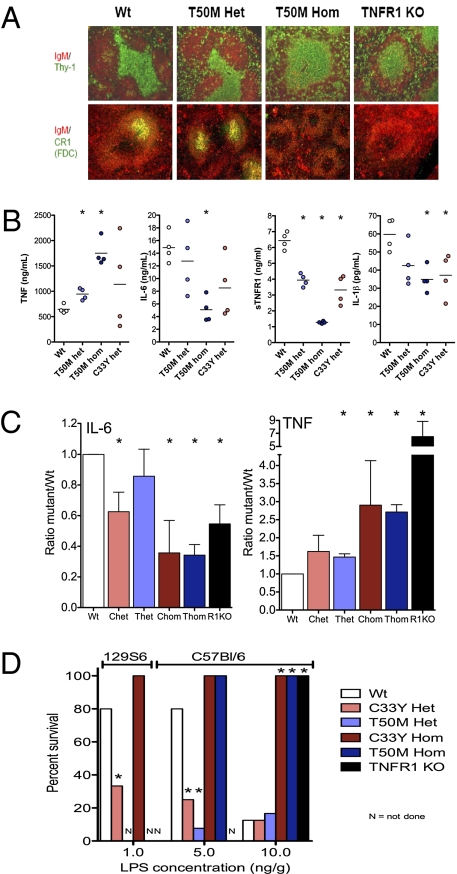
Fig. 3.
Phenotype and altered responsiveness to LPS in knockin mice harboring TRAPS-associated TNFR1 mutations. (A) Immunofluorescence images of the indicated markers in spleens from mice with targeted TNFR1 mutations, performed as previously described (38). Note the lack of well-defined T-cell zones or complement receptor 1 (CR1)-positive follicular dendritic cells in T50M TNFR1 homozygous mutant mice. (B) Serum cytokine concentrations 2 h after i.p. injection with 50 ng/g LPS in mice that were either WT (Wt), heterozygous for T50M mutation (T50M Het), homozygous for T50M mutation (T50M Hom), or heterozygous for the TNFR1 T50MC33Y mutation (C33Y Het). (C) Summary of serum concentrations of IL-6 and TNF 2 h after i.p. LPS injection from seven independent experiments performed as described in B. Ratios are normalized to the average of each cytokine detected in each experiment ± SEM. *, P < 0.05 from t test comparisons vs. WT mice. Chet, heterozygous C33Y; Chom, homozygous C33Y; R1KO, homozygous knockout for TNFR1; Thet, heterozygous T50M; Thom, homozygous T50M. (D) Summary of three independent lethality studies, with survival of mice of the indicated genotype (either 129S6 background or C57BL/6 background) after i.p. injection of the indicated doses of LPS and D-galactosamine. *, P < 0.05 from t test comparisons vs. WT mice.
Given the hyperresponsiveness of cells harboring TRAPS-associated TNFR1 mutations to TLR4 signaling, we reasoned that TNFR1-knockin mice might exhibit similar hypersensitivity to LPS in vivo. After i.p. challenge with LPS, we observed increased serum TNF concentrations in heterozygous T50M and C33Y TNFR1-mutant mice compared with WT controls. This increase was greater in homozygous than heterozygous TNFR1-mutant mice and also was seen in TNFR1- deficient mice (Fig. 3 B and C). Increased serum TNF could be caused either by increased production of TNF or by reduced soluble TNFR1 (sTNFR1). Measurement of sTNFR1 with an ELISA specific for the WT receptor showed progressively decreased levels of sTNFR1 in heterozygous and homozygous TNFR1-knockin mice treated with LPS (Fig. 3B). Thus, some of the increase in serum TNF may be caused by decreased sTNFR1, because the soluble receptor can shorten the circulating half-life of TNF (23, 24). In contrast to TNF, LPS-induced IL-6 and IL1β production progressively decreased in heterozygous and homozygous T50M and C33Y TNFR1-mutant mice compared with WT controls (Fig. 3 B and C). This decrease may result from impaired sensing of TNF in the liver, where the bulk of IL-6 is made, in response to LPS-induced TNF sensed by TNFR1 on hepatocytes (25, 26). Homozygous T50M- and C33Y-mutant mice produced significantly more TNF and less IL-6 than controls, whereas more modest changes were observed in heterozygous mice (Fig. 3C).
To determine the impact of TNFR1 mutations on the systemic response to LPS, we examined temperature responses to LPS injection and LPS-induced septic shock in TNFR1-mutant mice. LPS injection induced acute hypothermia in WT mice, peaking at 2.5 °C within 2 h of injection, similar to previous reports (27) (Fig. S7). Heterozygous T50M TNFR1-mutant mice had a significantly exaggerated and prolonged temperature drop consistent with hyperresponsiveness to LPS in vivo, whereas homozygous mice had a somewhat blunted acute hypothermic response (Fig. S7). After injection of LPS with D-galactosamine, which sensitizes mice to TNF-dependent endotoxic shock (26, 28), homozygous T50M and C33Y TNFR1-mutant mice and TNFR1-deficient mice were completely resistant to LPS-induced lethality (Fig. 3D). However, in heterozygous TNFR1-mutant mice, lethality was 70% at 5 ng/g LPS, a dose that induced mortality in less than 25% of WT mice. At higher doses of LPS, where WT mice have a mortality of more than 70%, lethality in heterozygous TNFR1-mutant mice is similar to that in WT mice. On a 129 genetic background, mice generally were more sensitive to LPS. C33Y heterozygous mice showed increased lethality in response to LPS at 1 ng/g, but homozygous C33Y TNFR1 mice remained completely resistant. TNF–TNFR1 interactions, rather than IL-6 or IL-1β feedback, have been found to be necessary for the acute hypothermia and lethality induced by LPS in mice (29, 30). Only the heterozygous TNFR1-mutant mice produce elevated levels of TNF and have an intact TNFR1 with which to sense it, illustrating the role of the WT TNFR1 in completing the pathogenic loop in TNFR1-mutant mice and paralleling the need for WT TNFR1 for maximal cytokine production by TNFR1-mutant macrophages.
TRAPS Patient PBMC Are Hyperresponsive to Low-Dose LPS.
Multiplex measurements of serum cytokine levels in a cohort of TRAPS patients who were not having a clinical flare at the time of sample collection revealed elevated levels of IL-6, TNF, and the proinflammatory chemokine IL-8 (Fig. S8A). IL-6 was most consistently elevated in TRAPS, as has been previously reported (31). These cytokines were elevated in patients with both structural TNFR1 mutations and nonstructural variants. Many patients with structural mutations had elevated C-reactive protein (CRP) levels higher than 2 mg/dL, even in the absence of clinical symptoms, and elevation in CRP did not correlate with serum IL6, TNF, or IL-8 levels (Fig. S8B).
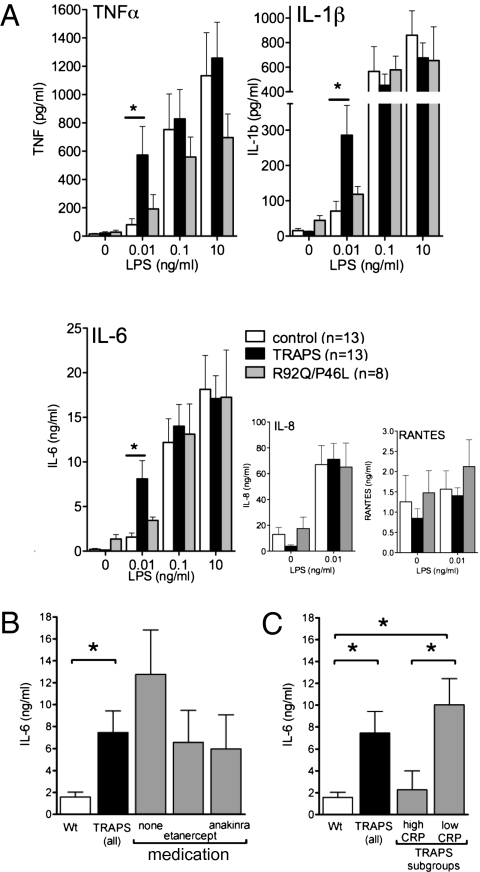
When PBMC from TRAPS patients were stimulated with LPS, at 0.1 and 10 ng/mL, comparable amounts of IL-1β, IL-6, and TNF were produced by cells from TRAPS patients and controls. However, at 0.01 ng/mL, a dose that did not induce significant cytokine production by normal cells, LPS induced exaggerated production of IL-1β, IL-6, and TNF, but not the chemokines IL-8/CXCL-8 or RANTES/CCL5, in cells from TRAPS patients harboring structural TNFR1 mutations (Fig. 4A). PBMC from patients harboring nonstructural TNFR1 variants (R92Q and P46L) produced levels of IL-1β, IL-6, and TNF similar to those produced by control cells at all LPS concentrations (Fig. 4A). TRAPS patients who were treated with anticytokine therapy at the time of sample collection had a trend toward lower cytokine production compared with the entire group, although levels in these patients still were higher than in controls (Fig. 4B). Interestingly, cytokine hyperproduction was blunted in asymptomatic patients with elevated serum concentrations of CRP (>2 mg/dL) at the time of sample collection (Fig.4C). Taken together, these results identify hyperproduction of IL-1β, IL-6, and TNF in response to low-dose LPS as a “cytokine signature” in PBMC from patients harboring structural TFNR1 mutations, consistent with the results from the TNFR1-knockin mice.
Fig. 4.
PBMC from TRAPS patients are hyperresponsive to low-dose LPS. (A) PBMC from patients with TRAPS heterozygous for structural TNFR1 mutations (n = 13, black bars), R92Q or P46L functional polymorphisms (n = 8, gray bars), or healthy controls (n = 13, white bars) were stimulated with the indicated dose of LPS for 24 h, and cytokine concentrations in supernatants were measured by cytometric bead array. P values obtained for differences vs. controls for samples from patients with structural TNFR1 mutations at 0.01 ng/mL LPS were 0.038 for TNF; 0.034 for IL-1β; and 0.011 for IL-6 (Student's unpaired t test). (B) Data from A were reanalyzed in subsets of patients treated with the indicated biologic agents. (C) Data from A were reanalyzed for patients having low (<2 mg/dL) or high (>2 mg/dL) CRP at the time of sample collection. *, P < 0.05 for the indicated comparisons.
Discussion
These results provide a mechanism by which the mutant TNFR1 can predispose to inflammation in TRAPS (Fig. S9). Although it does not function as a receptor for TNF, the mutant TNFR1 protein accumulates intracellularly and activates JNK and p38 signaling (Fig S9A). This activation sensitizes cells to the effects of other innate immune stimuli such as LPS, resulting in enhanced production of inflammatory cytokines and chemokines at low doses of such stimuli. In cells such as MEF, which do not produce TNF, cytokine hyperproduction is independent of the WT TNFR1 allele (Fig S8B). However, in immune cells that produce TNF, full-blown excessive inflammation is seen only in heterozygous TNFR1-mutant cells that still express the normal TNFR1 allele (Fig. S8C). Mice challenged with LPS also displayed a requirement for the WT TNF receptor in the enhanced lethality conferred by the mutant TNFR1 allele. Our results illustrate aspects of TNFR1 signaling, including (i) the ability of intracellularly retained receptors to signal in a ligand-independent fashion, and (ii) functional cooperation between a disease-causing mutant receptor and its WT counterpart in producing a dominantly inherited genetic disease. The cooperativity between WT and mutant receptors must be functional and indirect because the two receptors do not interact physically (16).
Rather than leading to the degradation of the mutant receptor, we find here that structural TNFR1 mutants associated with TRAPS accumulate to abnormally high levels. Some misfolded pro-teins linked to genetic diseases, such as the cystic fibrosis transmembrane receptor ΔF508 mutant, can be recognized by ER resident chaperones and efficiently degraded, whereas others, such as the misfolded mutants of superoxide dismutase associated with amyotrophic lateral sclerosis, accumulate and can cause cellular toxicity through an increased ER stress response (32). TRAPS-associated mutant TNFR1 does not appear to elicit either of these responses but does lead to activation of JNK and p38 MAPK. These abnormalities may result from spontaneous signaling by the mutated TNFR1 or could be induced indirectly through other mediators such as reactive oxygen species, which can sustain MAPK signaling through inactivation of MAPK phosphatases (21).
Our data with LPS-induced inflammation in TNFR1-mutant mice are consistent with a model in which the mutated TNFR1 synergizes with LPS to enhance initial rises in TNF, which then is sensed by WT TNFR1 in the liver, resulting in IL-6 and IL-1β production. WT TNFR1 was important in perpetuating a positive cytokine feedback loop that resulted in lethality, because only heterozygous, not homozygous, TNFR1-mutant mice exhibited hypersensitivity to LPS. Reduced levels of soluble TNFR1 also may play a role in propagating the systemic inflammation in TRAPS, because the mutant TNFR1 is unable to be secreted (16), and heterozygous TNFR1-mutant mice appear to be haploinsufficient in their production of soluble TNFR1 after LPS challenge (Fig. 4B). Mice homozygous for TRAPS-associated TNFR1 mutations phenocopy TNFR1-deficient mice in their resistance to LPS-induced sepsis and lack of germinal centers and follicular dendritic cell networks in the spleen. The T50M and C33Y TNFR1-mutant mice that model TRAPS do share some similarities with mice engineered to delete the cleavage site of TNFR1 (33), such as hyperresponsiveness to LPS. However, there are a number of important differences, because the cleavage-defective TNFR1 protein still functions as a surface receptor for TNF, whereas the TRAPS mutant TNFR1 does not. T50M or C33Y TNFR1-mutant mice do not develop the chronic active hepatitis seen in heterozygous or homozygous cleavage-defective mutant mice, and homozygotes for the cleavage-defective TNFR1 allele are more severely affected than heterozygous mice.
Exaggerated responses to low-dose LPS fit with the clinical features of TRAPS, in which trivial stimuli can provoke a clinical episode of fever and other inflammatory symptoms. The array of cytokines produced in excess by the cells in TRAPS patients exposed to LPS is different from those seen in patients with other autoinflammatory diseases. For example, patients with NALP3/cryopyrin mutations associated with the cryopyrinopathies mainly produce excess IL-1β and IL-1β−dependent cytokines (34). The blunted hyperinflammatory responses in PBMC from patients with elevated CRP suggest that counterregulatory antiinflammatory mechanisms known to occur during acute inflammatory episodes (35, 36) may still be intact in TRAPS. These mechanisms may aid in the resolution of TRAPS clinical flares. Taken together, the re-sults with TRAPS patient PBMC suggest that the inflammatory responses seen in TRAPS after minimal stimuli may stem from heightened sensitivity to initiating agents rather than to the absence of a ‘brake’ on the inflammatory cascade.
Our findings also may explain the partial responses to TNF blockade seen in TRAPS patients. We and others have observed recurrent disease flares and elevated acute-phase markers after initiation of TNF blockade in some patients (15, 37). TNF blockade inhibits only the component of inflammation dependent on TNF feedback through the WT TNFR1 but would not affect the TNF-independent components of the TRAPS phenotype that we have identified here. This effect is in contrast to IL-1 blockade in the cryopyrinopathies, which induces rapid and nearly complete resolution of inflammatory symptoms (34). Although it generally does not result in complete resolution of symptoms as it does in cryopyrinopathies, IL-1 blockade also is efficacious in some TRAPS patients, including those who do not respond to TNF blockade. Because IL-6 was one of the most consistently elevated cytokines produced by TNFR1-mutant cells and in the serum of TNFR1-mutant mice and TRAPS patients, IL-6 blockade might be an interesting alternative avenue of therapy to consider in TRAPS. Elucidating the biochemical mechanism by which the mutant TNFR1 activates MAPK signaling should provide new therapeutic targets for therapy in TRAPS. If the WT TNFR1 became retained intracellularly as it is in TRAPS, TNF-independent inflammation also might ensue in patients without mutations in TNFR1.
Materials and Methods
A full discussion of methods is given in SI Materials and Methods.
Patients and controls were included after informed consent under the approved National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) clinical research protocol 94-AR-0105. TNFR1-mutant mice were generated using a targeting vector containing nucleotide mutations to produce the T50M and C33Y mutation in the mouse tnfrsf1a gene (Fig. S1). PBMC from patients or peritoneal macrophages harvested from unmanipulated TNFR1-mutant mice were incubated in DMEM plus 10% heat-inactivated FCS, with or without indicated concentrations of lipopolysaccharide (Ultrapure Salmonella Minnesota R595; List Biological Laboratories Inc.). TNFR1-knockout mice on a C57BL/6 background (tm1Imx) were obtained from Jackson Laboratory. Cytokine analysis was performed using BD Biosciences or Bio-Rad cytokine bead arrays. For LPS challenge, mice received an i.p. injection of LPS (Escherichia coli 0127:B8 LPS; Sigma L3129), at the indicated concentrations, or a combination of E. coli LPS and 20 mg/kg D-galactosamine (Sigma G0500). Quantitative RT-PCR was performed on total RNA on a Prism 7700 ABI sequence detection system using primers and probes purchased from Applied Biosystems and were normalized to β2-microglobulin or 18S rRNA. NF-κB activation was analyzed and quantified by measuring ELISA-based DNA binding activity of p65 using a TransAm transcription factor assay kit (Active Motif). Cell survival was assayed by measuring mitochondrial dehydrogenase activity via method of transcriptional and translational (MTT) assay (ATCC). For temperature measurement in mice, body temperature was recorded every 30–60 min, with ambient room temperature maintained at 30 °C with a 12-h light/dark cycle, using surgically implanted PDT4000 E-mitter temperature transmitters (Mini Mitter Company).
Supplementary Material
Acknowledgments
We thank Beverly Barham and John Ryan for clinical support, Mihan Lee for technical assistance, Uli Siebenlist and Zheng-Gang Liu for reagents and helpful advice, and Robert Colbert and Massimo Gadina for critical reading of the manuscript. This research was supported by intramural research funding from National Institute of Arthritis and Musculoskeletal and Skin Diseases. A.S. is supported by a Netherlands Organization for Health Research and Development (ZonMW) Veni grant. A.J.J. and R.M. were supported by the Howard Hughes Medical Institute-National Institutes of Health Scholars Program, and A.C.B. was supported by the National Institutes of Health Clinical Research Training Program, a public–private partnership between the Foundation for the National Institutes of Health and Pfizer Inc.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914118107/-/DCSupplemental.
References
- 1.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MF, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 4.Kimberley FC, Lobito AA, Siegel RM, Screaton GR. Falling into TRAPS - receptor misfolding in the TNF receptor 1-associated periodic fever syndrome. Arthritis Res Ther. 2007;9:217. doi: 10.1186/ar2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 6.McGonagle D, McDermott MF. A proposed classification of the immunological diseases. PLoS Med. 2006;3:e297. doi: 10.1371/journal.pmed.0030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touitou I, et al. Infevers: An evolving mutation database for auto-inflammatory syndromes. Hum Mutat. 2004;24:194–198. doi: 10.1002/humu.20080. [DOI] [PubMed] [Google Scholar]
- 9.Tchernitchko D, et al. Unexpected high frequency of P46L TNFRSF1A allele in sub-Saharan West African populations. Eur J Hum Genet. 2005;13:513–515. doi: 10.1038/sj.ejhg.5201344. [DOI] [PubMed] [Google Scholar]
- 10.Ravet N, et al. Clinical significance of P46L and R92Q substitutions in the tumour necrosis factor superfamily 1A gene. Ann Rheum Dis. 2006;65:1158–1162. doi: 10.1136/ard.2005.048611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattorno M, et al. A diagnostic score for molecular analysis of hereditary auto-inflammatory syndromes with periodic fever in children. Arthritis Rheum. 2008;58:1823–1832. doi: 10.1002/art.23474. [DOI] [PubMed] [Google Scholar]
- 12.Huggins ML, et al. Shedding of mutant tumor necrosis factor receptor superfamily 1A associated with tumor necrosis factor receptor-associated periodic syndrome: Dif-ferences between cell types. Arthritis Rheum. 2004;50:2651–2659. doi: 10.1002/art.20380. [DOI] [PubMed] [Google Scholar]
- 13.Hull KM, et al. The TNF receptor-associated periodic syndrome (TRAPS): Emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81:349–368. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Arostegui JI, et al. Etanercept plus colchicine treatment in a child with tumour necrosis factor receptor-associated periodic syndrome abolishes auto-inflammatory episodes without normalising the subclinical acute phase response. Eur J Pediatr. 2005;164:13–16. doi: 10.1007/s00431-004-1563-1. [DOI] [PubMed] [Google Scholar]
- 15.Jacobelli S, Andre M, Alexandra JF, Dode C, Papo T. Failure of anti-TNF therapy in TNF receptor 1-associated periodic syndrome (TRAPS) Rheumatology (Oxford) 2007;46:1211–1212. doi: 10.1093/rheumatology/kel298. [DOI] [PubMed] [Google Scholar]
- 16.Lobito AA, et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS) Blood. 2006;108:1320–1327. doi: 10.1182/blood-2005-11-006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todd I, et al. Mutant tumor necrosis factor receptor associated with tumor necrosis factor receptor-associated periodic syndrome is altered antigenically and is retained within patients' leukocytes. Arthritis Rheum. 2007;56:2765–2773. doi: 10.1002/art.22740. [DOI] [PubMed] [Google Scholar]
- 18.Rebelo SL, et al. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 2006;54:2674–2687. doi: 10.1002/art.21964. [DOI] [PubMed] [Google Scholar]
- 19.Todd I, et al. Mutant forms of tumour necrosis factor receptor I that occur in TNF-receptor-associated periodic syndrome retain signalling functions but show abnormal behaviour. Immunology. 2004;113:65–79. doi: 10.1111/j.1365-2567.2004.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 21.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 22.Tang F, et al. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan KC, et al. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: Identification of a novel in vivo role for p75. J Exp Med. 1995;181:607–617. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peschon JJ, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 25.Bultinck J, Brouckaert P, Cauwels A. The in vivo contribution of hematopoietic cells to systemic TNF and IL-6 production during endotoxemia. Cytokine. 2006;36:160–166. doi: 10.1016/j.cyto.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Rothe J, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 27.Blanque R, Meakin C, Millet S, Gardner CR. Hypothermia as an indicator of the acute effects of lipopolysaccharides: Comparison with serum levels of IL1 beta, IL6 and TNF alpha. Gen Pharmacol. 1996;27:973–977. doi: 10.1016/0306-3623(95)02141-8. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein R. D-galactosamine lethality model: Scope and limitations. J Endotoxin Res. 2004;10:147–162. doi: 10.1179/096805104225004879. [DOI] [PubMed] [Google Scholar]
- 29.Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–R277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- 30.Remick D, et al. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock. 1995;4:89–95. doi: 10.1097/00024382-199508000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Nowlan ML, et al. Systemic cytokine levels and the effects of etanercept in TNF receptor-associated periodic syndrome (TRAPS) involving a C33Y mutation in TNFRSF1A. Rheumatology (Oxford) 2006;45:31–37. doi: 10.1093/rheumatology/kei090. [DOI] [PubMed] [Google Scholar]
- 32.Bruijn LI, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 33.Xanthoulea S, et al. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J Exp Med. 2004;200:367–376. doi: 10.1084/jem.20040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Deuren M, et al. Elective orthopedic surgery, a model for the study of cytokine activation and regulation. Cytokine. 1998;10:897–903. doi: 10.1006/cyto.1998.0367. [DOI] [PubMed] [Google Scholar]
- 36.van Deuren M, et al. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Dis. 1994;169:157–161. doi: 10.1093/infdis/169.1.157. [DOI] [PubMed] [Google Scholar]
- 37.Bodar EJ, Drenth JP, Simon A, van der Meer JW. Ned Tijdschr Geneeskd. 2007;151:836–837. author reply 837. [PubMed] [Google Scholar]
- 38.Ettinger R, et al. A critical role for lymphotoxin-beta receptor in the development of diabetes in nonobese diabetic mice. J Exp Med. 2001;193:1333–1340. doi: 10.1084/jem.193.11.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.