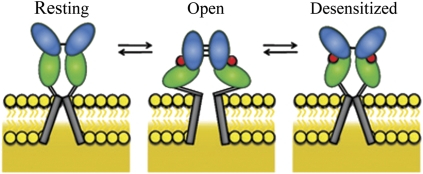
Fig. 4.
Proposed mechanism showing changes in the ligand-binding domain associated with the resting state, activation, and desensitization. Agonist binding to the ligand-binding domain induces cleft closure in the ligand-binding domain, pulling apart the linker to the channel segments opening the channel (activation). The open-channel form is transiently stabilized through transiently formed dimer interface interactions at the ligand-binding domain. Stress on the linker domain eventually results in decoupling of the ligand-binding domain dimer interface interactions, leading to channel closure (desensitization).