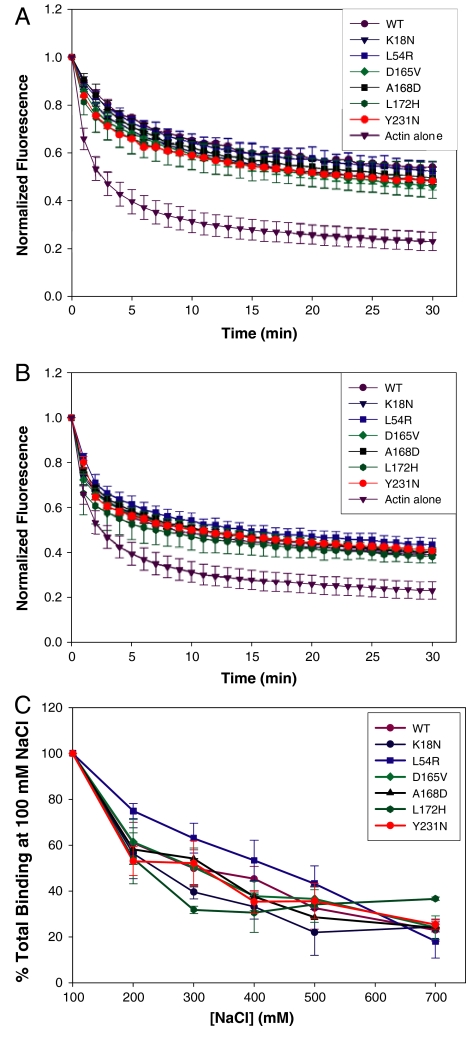
Fig. 5.
Effect of WT and mutant dystrophin on actin depolymerization. (A and B) WT and mutant dystrophins were coincubated with actin filaments at dystrophin:actin ratios of 1∶12 (A) and 1∶24 (B). Filament depolymerization was induced by dilution and monitored by decay of pyrene-actin fluorescence. Normalized fluorescence is plotted as a function of time (minutes). (C) Effect of NaCl concentration on WT and mutant dystrophin binding to actin. WT and mutant dystrophins at a concentration of 0.5 μM were subjected to high-speed cosedimentation with Kd concentrations of F-actin at the indicated concentration of NaCl. The data are expressed as the percent of each protein pelleted in the presence of 100 mM NaCl. (A–C) Error bars represent S.E.M.