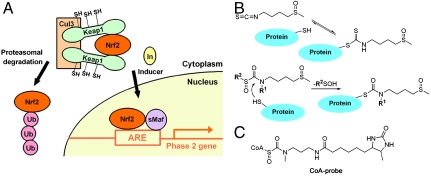
Fig. 1.
Proposed chemoprotective mechanism of sulforaphane for phase 2 gene activation involves covalent Cys modification of Keap1. (A) Mechanism of phase 2 enzyme induction. In the basal state, the transcription factor Nrf2 is efficiently ubiquitylated and targeted for proteasomal degradation by forming a complex with Keap1 and Cul3 E3 ligase. Inducers such as sulforaphane modify cysteines of Keap1, which leads to stabilization and translocation of Nrf2 to the nucleus, and its binding to ARE and stimulation of phase 2 gene transcription. (B) Reactions of sulforaphane and sulfoxythiocarbamate analog with a thiol. The isothiocyanate group of sulforaphane reacts with sulfhydryl groups on protein to form dithiocarbamate adduct that appear to be kinetically labile (Top), whereas sulfoxythiocarbamate analog forms a relatively stable thiocarbamate derivative (Bottom). (C) Structure of sulfoxythiocarbamate CoA probe developed previously (24).