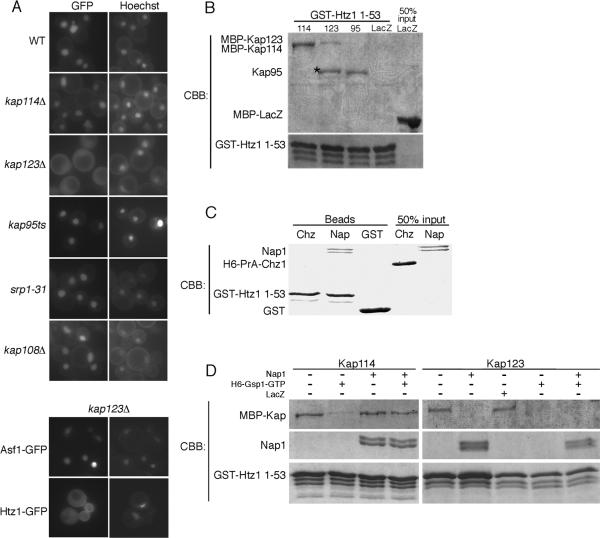
Figure 3.
Htz1, Nap1, Kap114 form a co-complex. (A) The Htz1 NLS (residues 1–53) or Asf1 GFP2 reporter was expressed in yeast strains containing mutations of individual KAP genes as indicated and localized by fluorescent microscopy. Coincident Hoechst staining is shown. (B) Recombinant GST-Htz11–53 (250 nM) was immobilized on glutathione sepharose beads and incubated with the indicated recombinant Kap protein (MBP-Kap114 100 nM, MBP-Kap123 500 nM, Kap95 500 nM). MBP-LacZ (500 nM) was utilized as a negative control. After extensive washing, bound protein was analyzed by SDS-PAGE and Coomassie blue staining (CBB). A 50% input of MBP-LacZ is shown. * indicates a proteolytic product of MBP-Kap123. (C) Recombinant GST-Htz11–53 (400 nM) was immobilized on glutathione sepharose beads and incubated with either recombinant Nap1 (250 nM) or H6-PrA-Chz1 (500 nM). Similarly, GST (500 nM) was incubated with Nap1 (250 nM) as a control. After washing, protein was eluted from the beads and analyzed by SDS-PAGE and Coomassie stain. 50% input of Nap1 and H6-PrA-Chz1 are indicated. (D) Recombinant GST-Htz11–53 (400 nM) was immobilized on glutathione sepharose in the presence or absence of Nap1 (250 nM). MBP-Kap114 (100 nM) or MBP-Kap123 (300 nM) was pre-incubated with or without H6-Gsp1Q71L-GTP (50 μM) and/or MBP-LacZ (400nM) and then added to the binding reaction. After extensive washing, bound protein was analyzed by SDS-PAGE and Coomassie stain.