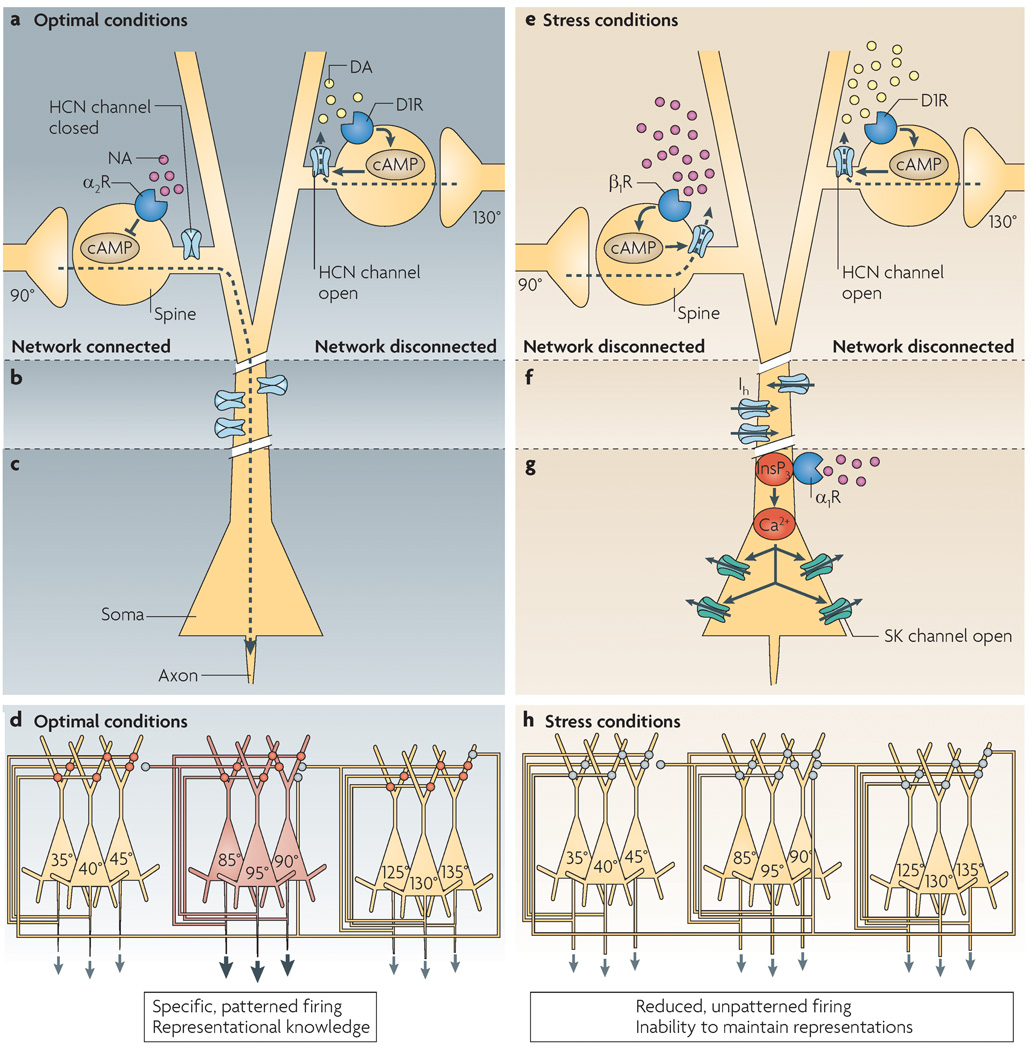
Figure 4. A working model showing how prefrontal cortex pyramidal cell activity might be regulated under optimal versus stress conditions.
a | The efficacy of network inputs onto dendritic spines of the apical and/or basal dendrites is dynamically regulated by cyclic AMP–hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN channel) signalling. Under optimal conditions, appropriate network connections are strengthened by α2A-receptor inhibition of cAMP–HCN channel signalling, whereas inappropriate network connections are weakened by dopamine D1 receptor activation of cAMP–HCN channel signalling. The small volume of the spine compartment probably allows very localized regulation of nearby HCN channels. The resulting specific pattern of network connectivity allows accurate representation of the cue’s spatial position in the oculomotor delayed response task, and the breadth of connectivity can be dynamically regulated based on current cognitive demands. b | HCN channels on the plasma membrane of dendritic shafts probably regulate excitability. They might not be open when the dendrite is depolarized by large numbers of excitatory network connections. c | Under optimal conditions, the cell body probably receives network signals and fires accordingly. d | Under optimal conditions the PFC shows highly patterned activity, whereby specific subsets of neurons are active to represent the precise spatial position of a stimulus. This is denoted in the figure, in which only the microcircuit representing 90° is active. Note that interneurons are not shown in this diagram for the sake of clarity. Network inputs are shown on the apical dendrites, but they might actually be on basal dendrites. Red circles represent α2A-receptor strengthening of network inputs; grey circles represent D1 receptor–cAMP weakening of a network connection. e | Under conditions of stress, high levels of cAMP open HCN channels throughout the dendrite, weakening all network connections. f | With loss of network excitatory inputs the dendrite might hyperpolarize, and HCN channels on dendritic shafts might open to help maintain dendritic excitability. HCN channel opening would also reduce the effectiveness of inputs onto the distal portions of the dendrite. g | High levels of phosphatidylinositol signalling during stress would increase inositol-1,4,5-trisphosphate (InsP3)-mediated waves of intracellular Ca2+ release. When the Ca2+ waves invade the soma, they open small-conductance, Ca2+-activated K+ channels (SK channels) and reduce cell firing. h | Under stressful conditions, network connections are weakened (grey circles) and, as a result, network firing is reduced and unpatterned. Thus, prefrontal cortex neurons are unable to accurately represent information in working memory. Unpatterned, generalized activity might serve another function — for example, stimulating stress pathways in the brainstem.