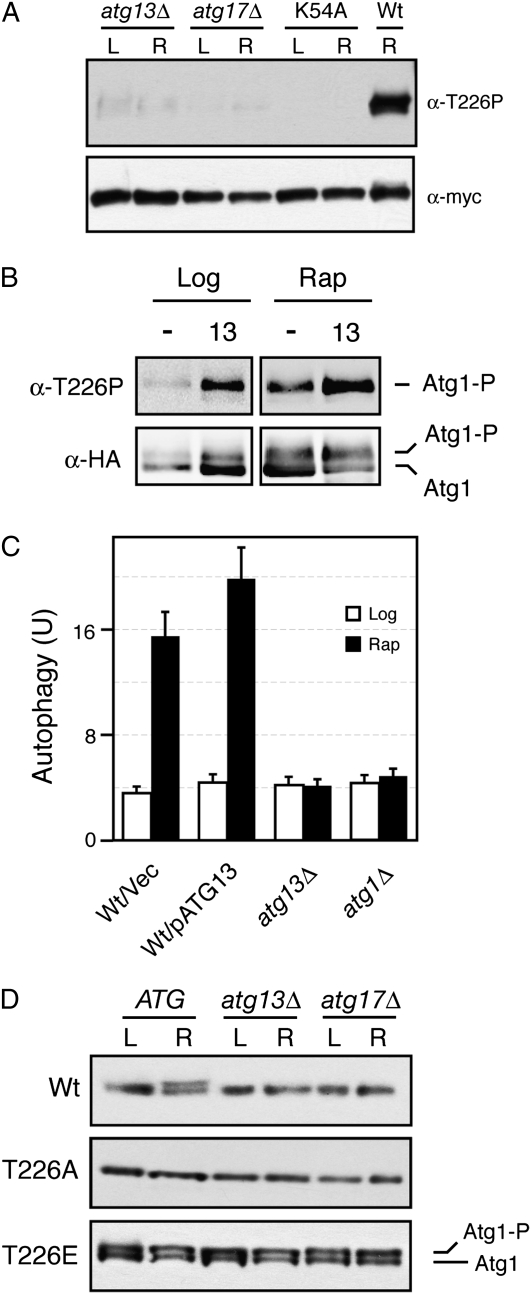
Figure 10.—
Atg13 and Atg17 were required for Atg1 autophosphorylation within the activation loop. (A) T226 phosphorylation in atg13Δ and atg17Δ mutants. The wild-type Atg1 was precipitated from atg13Δ, atg17Δ, or wild-type cells and analyzed by Western blotting with the anti-T226P phosphospecific antibody. The Atg1K54A variant (K54A) was used as a kinase-defective control. L, log phase; R, rapamycin-treated. (B) Overexpression of Atg13 resulted in an increased level of T226 phosphorylation. The relative level of T226 phosphorylation was assessed in both log-phase and rapamycin-treated wild-type cells carrying either a vector control (−) or an ATG13 overexpression plasmid. The wild-type Atg1 was immunoprecipitated from these cells with an antibody specific for the HA epitope present on this protein. (C) Overexpression of Atg13 resulted in elevated levels of autophagy. A plasmid overexpressing Atg13 or a vector control were introduced into wild-type cells, and autophagy levels were assessed with the Pho8Δ60 ALP-based assay. The cells were treated with 200 ng/ml rapamycin for 0 (Log) or 4 hr (Rap) before analysis. (D) Atg13 and Atg17 were not required for the autophosphorylation of Atg1T226E in vivo. Cell extracts were prepared from either log-phase (L) or rapamycin-treated (R) cultures of wild-type, atg13Δ, and atg17Δ cells carrying the indicated Atg1 proteins. The relative levels of the slower-migrating, autophosphorylated form of Atg1 were assessed by Western blotting with an anti-myc antibody. The Atg1 proteins assayed were all tagged with three copies of the myc epitope.