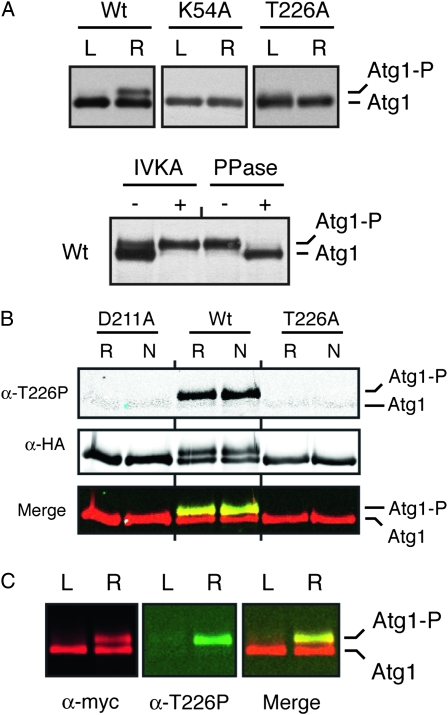
Figure 3.—
The Atg1T226A variant was defective for Atg1 autophosphorylation in vivo. (A) The presence of the T226A alteration resulted in a loss of Atg1 autophosphorylation in vivo. Western blots were performed with extracts prepared from either log-phase (L) or rapamycin-treated (R) cells with the indicated Atg1 proteins. In the bottom part, immunoprecipitated wild-type Atg1 was either mock treated (IVKA−) or subjected to an in vitro autophosphorylation reaction with 3 mM ATP (IVKA+). An aliquot of this latter sample was then split into two and either mock treated (PPase−) or incubated with λ phosphatase (PPase+). PPase, λ phosphatase; IVKA, in vitro kinase assay; Atg1-P, autophosphorylated form of Atg1 that exhibits an altered mobility on SDS-polyacrylamide gels. (B) Atg1 activation loop phosphorylation was elevated in response to nitrogen starvation and required the presence of a functional Atg1 kinase domain. In B and C, the indicated Atg1 proteins were immunoprecipitated from cell extracts with an antibody directed against the indicated epitope, separated on SDS-polyacrylamide gels, and then analyzed by Western blotting with the indicated antibodies. The cell extracts were prepared from either log-phase (L), rapamycin-treated (R) or nitrogen starved (N) cultures. The protein signals were detected with a LI-COR Odessey infrared detection system. For nitrogen starvation, log-phase cells were transferred to SD-N medium for 6 hr. D211A, a kinase-defective variant of Atg1. (C) The anti-T226P phosphospecific antibody (α-T226P) recognized a slower-migrating, autophosphorylated form of Atg1 that appeared upon rapamycin treatment. The protein signals were detected with a LI-COR Odessey infrared detection system and the right-most part shows a merged image for the α-myc and α-T226P signals.