Fig. 15.
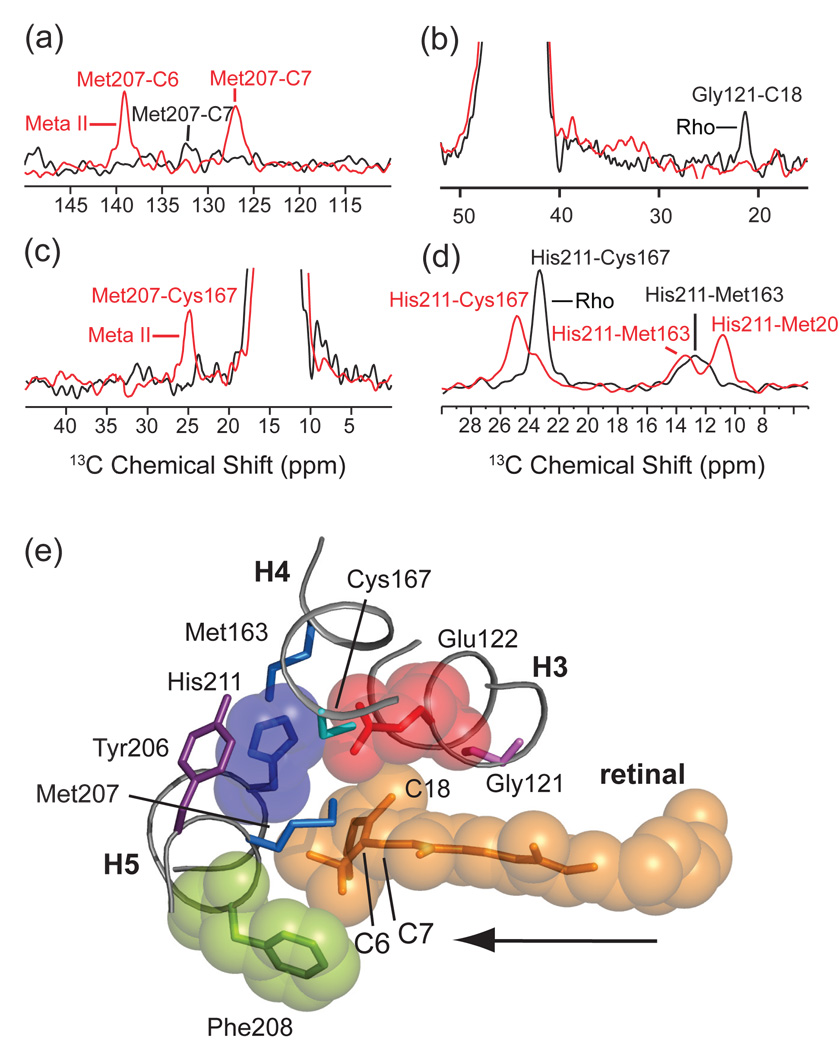
Molecular switches on the extracellular surface of rhodopsin: Glu122-His211. Motion of the retinal chromophore toward H5 is revealed by an increase in NMR cross peak intensity between the retinal and Met207 (a) and by a decrease in cross peak intensity between the retinal and Gly121 (b). Panel (a) presents rows through the diagonal resonance of 3Cε-Met207 in rhodopsin and Meta II from 150 MHz 13C 2D DARR NMR spectra. Panel (b) presents rows through the diagonal resonance of 13Cα-Gly121 in rhodopsin and Meta II from 150 MHz 13C 2D DARR NMR spectra. Rearrangement of the hydrogen bonding network centered on His211 is revealed by changes in internuclear distance between His211 and surround in amino acids. In panel (c), rows are presented from 150 MHz 13C 2D DARR NMR spectra taken through the diagonal of 13Cε1-His211 in rhodopsin at 136.9 ppm and Meta II at 137.5 ppm. In rhodopsin, cross peaks are observed with 13Cβ-Cys167 at 23.7 ppm and with 13Cε-Met163 at 13.1 ppm. In Meta II, cross peaks with 13Cβ-Cys167 are observed at 25.3 ppm, with Met207 at 13.8 ppm and with Met163 at 11.2 ppm. On conversion to Meta II, the His211-Cys167 contact weakens, whereas the His211 side chain packs closer to the Met207 side chain on H5. In panel (d), a new cross peak appears between 13Cε-Met207 and 13Cβ-Cys167 in the formation of Meta II. (e) Guided molecular dynamics simulations show that the retinal chromophore in rhodopsin shifts toward H5 in Meta II. The retinal β-ionone ring contacts Glu122 and Met207 and leads to a change in a hydrogen bonding network involving H3, H4 and H5. The formation of a direct interaction between Glu122 and His211 stabilizes the active Meta II state. The figure is adapted from Ref. [106].