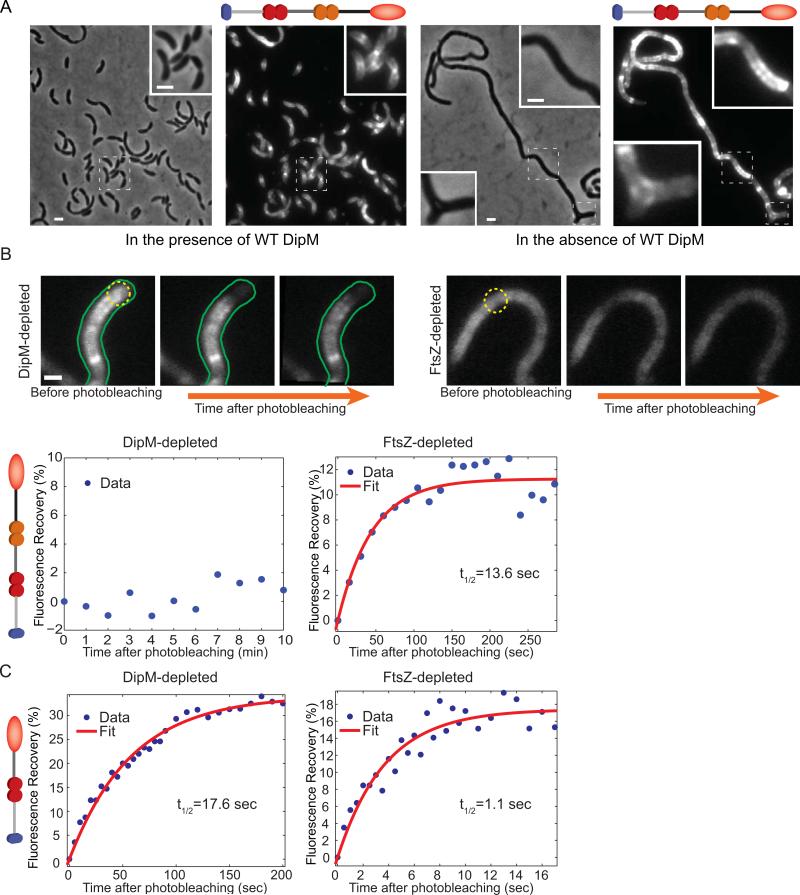
Fig. 5.
Localization and mobility of the DipM LysM tandems in FtsZ- and DipM-depleted cells. (A) Localization of the localization-proficient, inactive DipMΔ501-609-mCherry fusion in CJW3446 cells inducing the synthesis of wild-type DipM (growth in the presence of 250 μM of the vanillic acid inducer), or repressing the synthesis of wild-type DipM (growing without vanillic acid inducer). (B) FRAP experiments of DipMΔ501-609-mCherry (carrying two LysM tandems) in DipM- or FtsZ-depleted CJW3446 cells (n=21 and 20, respectively). Depletion of DipM or FtsZ was achieved by removing vanillic acid or xylose from the cultures for about 24 and 5 h, respectively. Regions within cell filaments were photobleached with a 1s laser pulse series. Top, examples of fluorescent images of DipMΔ501-609-mCherry in DipM- and FtsZ-depleted cells before and after photobleaching. The photobleached regions are indicated by yellow dotted circles. The scale bar corresponds to 1 μm. Bottom, the percentage of fluorescence recovery was plotted as a function of time following photobleaching. The average values and the best fit (see Material and methods) are shown in blue and red, respectively. (C) FRAP experiments of DipMΔ297-609-mCherry (carrying a single LysM tandem) in DipM- or FtsZ-depleted cells (CJW3445 and CJW3530, respectively). Regions of cell filaments were photobleached with a 300ms laser pulse series. Quantification was performed as in panel (B).