Fig. 6.
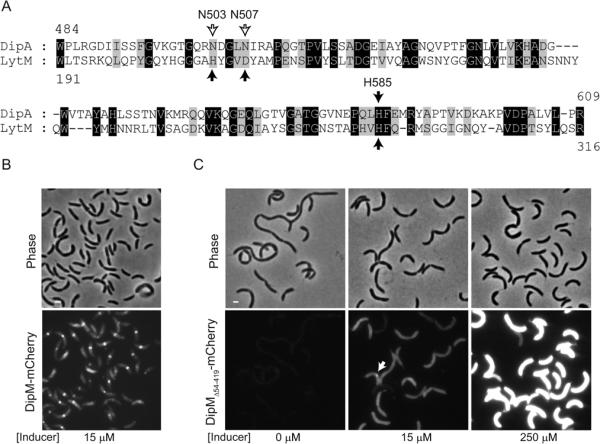
The non-canonical LytM domain of DipM can support function. (A) Alignment between S. aureus LytM catalytic domain and the non-canonical LytM domain of C. crescentus DipM. Black arrows indicate the conserved residues that are directly involved in metal coordination in the crystal structure of S. aureus LytM. The empty arrows indicate the non-conserved residues in DipM at positions of metal coordination. (B) Images of ΔdipM cells expressing wild-type dipM fused to mCherry (strain CJW3440). The cells were grown in the presence of 15 μM vanillic acid (inducer) for 5 h in M2G medium at 30°C to induce the synthesis of the mCherry fusions. Under these conditions, the level of expression of wild-type dipM allele was sufficient to fully suppress the cell filamentation phenotype caused by the ΔdipM mutation. (C) Images of ΔdipM cells carrying the non-canonical LytM domain of DipM (and no LysM domains) fused to mCherry (DipMΔ54–419-mCherry; strain CJW3444). Different levels in DipMΔ54–419-mCherry proteins were obtained by growing the cells in the presence of different concentrations of the vanillic acid inducer. The arrow indicates an example of the infrequent accumulation of DipMΔ54-419-mCherry signal at constriction sites.
