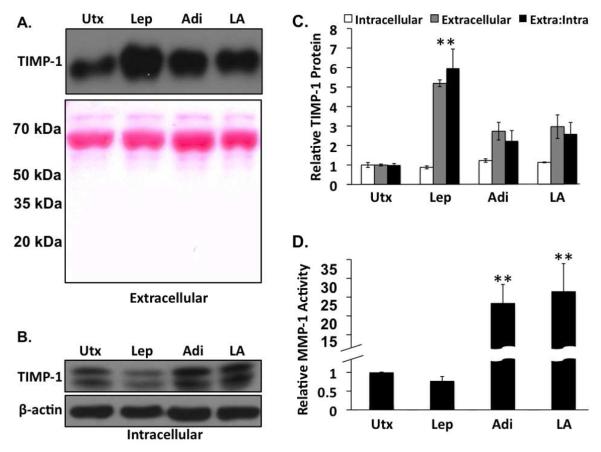
Figure 4. Adiponectin decreases tissue inhibitor of metalloproteinase (TIMP)-1 secretion in the presence of leptin and increases MMP-1 activity.
A) Western analysis of extracellular TIMP-1 from leptin (Lep), adiponectin (Adi), and co-treated (LA) rat HSCs. Ponceau S staining of the membranes was used as a protein loading control. B) Western analysis of intracellular TIMP-1 from leptin (Lep), adiponectin (Adi), and co-treated (LA) rat HSCs. β-actin was used as a protein loading control. (C) Quantitative representation of the relative intracellular (white bars), extracellular (gray bars) TIMP-1 protein levels, and the ratio of extracellular to intracellular (extra:intra, black bars) TIMP-1 protein from leptin (Lep), Adiponectin (Adi), or leptin and adiponectin (LA)-treated rat HSCs, D) Relative MMP-1 activity in rat HSCs was assessed by fluorimetric immunoassay. Adiponectin alone (Adi) or the co-administration of adiponectin and leptin (LA) resulted in a significant increase in MMP-1 activity compared to untreated samples (Utx) or leptin treatment alone (Lep) (**p < 0.01 vs. untreated).