Summary
We examined the utility of agar dilution to screen yeasts for reduced susceptibility to several newer antifungal drugs including echinocandins and azoles. We compared agar dilution susceptibility screening with the CLSI method for Candida isolates. CHROMagar Candida medium was prepared with echinocandins and azoles added independently to agar prior to solidification. Assessment of resistance was based on growth characteristics, wherein decreased colony size in the presence of antifungal drugs was used as an indicator of resistance. Clinical Candida isolates of C. albicans, C. glabrata, C. parapsilosis C. tropicalis, C. guilliermondii, C. lusitaniae, C. rugosa and C. dubliniensis were screened for drug susceptibility. Overall, antifungal susceptibility against anidulafungin, caspofungin, micafungin, posaconazole and voriconazole, determined using CHROMagar agar dilution, were shown to be 96, 80, 94, 90 and 97% accurate, respectively, within one tube dilution of CLSI MICs for these clinical isolates. Categorical errors by percentage, had a broader range. Major errors with anidulafungin, caspofungin and micafungin were 3, 6 and 0%, respectively, while very major errors were 15, 55 and 38%. Major errors with posaconazole and voriconazole, respectively, were 12 and 0%, while very major errors were 0 and 22% respectively, compared to CLSI standards. Most of the assessment errors were with C. glabrata and C. parapsilosis. Agar dilution screening for drug susceptibility with the current panel of antifungal drugs is rapid, accurate and effective, however, determination of resistance or non-susceptibility in yeasts may be more problematic, and may be species dependent.
INTRODUCTION
The Clinical and Laboratory Standards Institute (CLSI) formerly known as the National Committee for Clinical Laboratory Standards defines the standard methodology [1]. This method is not readily employed in routine clinical laboratories. However, a variety of alternate methods have been devised—Etest, disk diffusion, sensititre, and agar dilution [2–7]. We have previously shown the agar dilution method to be an effective screening tool to determine fluconazole susceptibility in Candida albicans and other yeasts [4,5]
Antifungal resistance varies significantly by yeast species. Amongst Candida spp., Candida albicans is typically susceptible to the azole class of antifungals, including fluconazole, while Candida krusei and Candida glabrata have demonstrated intrinsic resistance or dose dependent susceptibility, respectively, to fluconazole. Both C. glabrata and C. krusei show increased susceptibility in vitro to voriconazole [7] and posaconazole [3], although azole cross-resistance for C. glabrata is common. The in vitro activities of anidulafungin, micafungin and caspofungin, all recently approved echinocandins, against Candida falls into two groups. Candida albicans, C. glabrata and C. tropicalis are generally very susceptible to this class of drugs, with notable species expections, such as C. parapsilosis and C. guilliermondii which show decreased susceptibility [6,8]. Since the incidence of disease from non-C. albicans yeasts, in particular, Candida glabrata, is increasing [9,10], we evaluated the effectiveness of agar dilution as a screening tool for yeast susceptibility, including the newer azoles and the echinocandins [11–13].
MATERIALS and METHODS
Preliminary growth medium assessment
Seven isolates of Candida species with voriconazole and caspofungin MICs determined by CLSI broth microdilution were collected from stock cultures. One isolate of C. albicans with susceptiblity to both voriconazole and caspofungin, and two C. albicans, two C. glabrata, and two C. krusei, each being classified as resistant to voriconazole (MIC ≥4 μg/ml) or non-susceptible to caspofungin (MIC >2 μg/ml) were chosen for study. In preliminary studies, four types of yeast growth media were assessed for suitability as a medium for agar dilution screening with both an azole drug and an echinocandin: CHROMagar Candida (CHROMagar Company, Paris France), RPMI 1640 (Mediatech, Inc., Herndon, VA), Antibiotic Medium 3, (Becton, Dickinson and Co., Sparks, MD), and Sabouraud dextrose agar (Becton, Dickinson and Co.). Each was prepared according to manufacturer’s directions with the addition of voriconazole (Vfend; Pfizer Roerig, New York, NY) at concentrations of 0, 1, and 4 μg/ml. Similar preparations were made with the addition of caspofungin (Cancidas; Merck & Co., Inc., Whitehouse Station, NJ) at concentrations of 0, 0.5, and 1 μg/ml. At the time of this study, CLSI guidelines for susceptibility testing or breakpoints for resistance were not yet established for the echinocandins. In the absence of a defined breakpoint for caspofungin, 1 μg/ml was chosen to represent isolates that were considered less susceptible. Additionally, Bacto agar (Becton, Dickinson and Co.) was added to RPMI and Antibiotic medium 3 preparations at 15 g/l as previously described [4,5]. These media were dispensed (20 ml) into 100-mm-diameter petri dishes. Solidified plates were stored at 4oC for up to a week prior to use [4,14].
CHROMagar Candida
CHROMagar Candida was prepared from powdered medium according to the manufacturer’s instructions with the addition of posaconazole (Noxafil; Schering Corporation, Kenilworth, NJ) or voriconazole to give 1- and 4- μg/ml concentrations or anidulafungin (Eraxis, Pfizer Roerig), caspofungin, and micafungin (Mycamine; Astellas Pharma US, Inc., Deerfield, IL), to yield 0.5- and 1- μg/ml concentrations. Control plates without antifungal drugs were similarly prepared. The prepared medium, which contained chloramphenicol (0.5 g/l) and agar (15 g/l), was dispensed (20 ml) into 100 mm diameter petri dishes. Hardened plates were stored at 4oC for up to a week prior to use [4,14].
Susceptibility screening
CHROMagar Candida, a chromogenic medium, yields Candida-specific color patterns permitting presumptive identification, which can be confirmed using standard techniques, such as germ tube formation and carbohydrate assimilation patterns. Typically, C. albicans colonies are green in color, while C. krusei colonies are lavender and C. glabrata colonies have a purple appearance, as shown in Figure 1 [5]. In these series of experiments, conducted in duplicate, we spread 10 μl of yeast suspensions containing 103 CFU/ml, on sets of plates containing the two concentrations of each drug under study as well as onto control plates without drug, as shown in Figure 2.
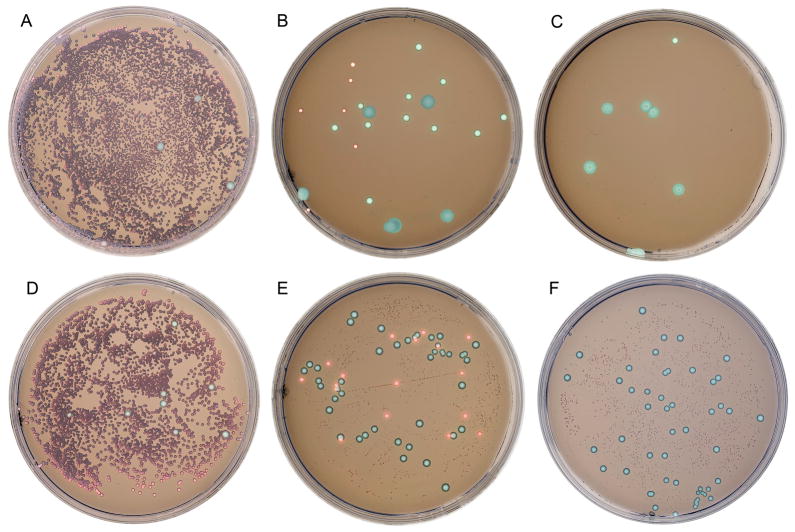
Figure 1.
Simulated clinical oral rinse sample on the CHROMagar Candida plates without drug (A,D), caspofungin concentrations of 0.5 (B) and 1 (C) μg/ml, and voriconazole concentrations of 1 (E) and 4 (F) μg/ml. The overwhelming numbers of a susceptible C. albicans isolate masked the resistant yeasts on drug-free plates, covering the whole plate with large green colonies (A,D). On plates with drug, the susceptible C. albicans did not grow normally, and yeasts with reduced susceptibility could be identified by color and morphology (B,C,E,F).
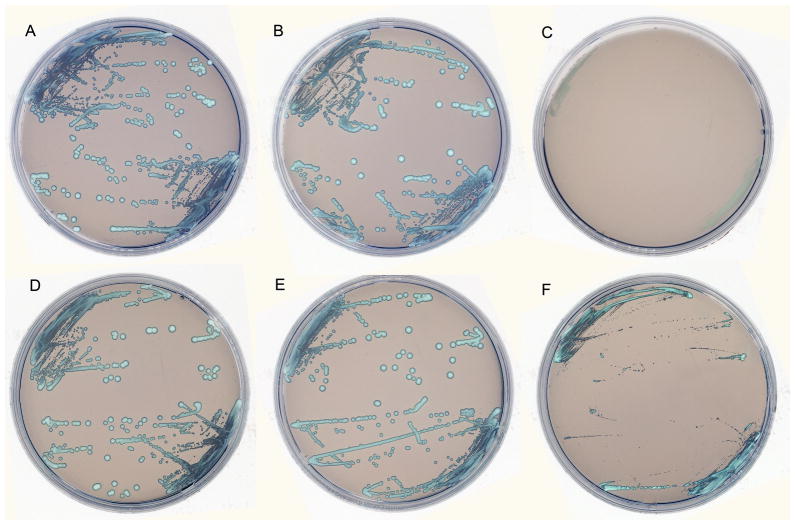
Figure 2.
C. glabrata on CHROMagar plates without caspofungin (A), with caspofungin 0.5 μg/ml (B), with caspofugin 1 μg/ml (C). C. glabrata on CHROMagar plates without voriconazole (D), with voriconazole 1 μg/ml (E), with voriconazole 4 μg/ml (F). Each isolate was plated in duplicate for each series. Both series A–C (caspofungin) and D–F (voriconazole) show susceptibility to the antifungal drug at the highest concentration, but not at the lower concentration.
On the plates without drug, yeast isolates, whether susceptible or not, grew normally and exhibited their CHROMagar specific colors. Results from the antifungal-containing media were recorded as susceptible or resistant on the basis of growth characteristics. Colonies that demonstrated suppressed growth on media with antifungal drugs, being visualized as having significantly decreased size (≤50%), as compared with growth on media without an antifungal agent were recorded as susceptible. Colonies that demonstrated growth that was indistinguishable on media with or without the appropriate antifungal agent were recorded as resistant (or non-susceptible) at that drug concentration [4,5]. These data were then compared to previously obtained MIC results for these same isolates using CLSI criteria for antifungal susceptibility testing.
Screening of C. glabrata
A collection of clinical C. glabrata isolates that had been previously submitted to the University of Texas Health Science Center (UTHSCSA) Fungus Testing Laboratory (San Antonio, TX) for MIC determination by CLSI methodology [1] were subcultured and evaluated blindly by the agar dilution method described above. These isolates were screened for susceptibility against caspofungin (n=74) and voriconazole (n=76). At the beginning of this study, MIC breakpoints for the echinocandins had not been established, and in these experiments, MICs for caspofungin <1 μg/ml were considered susceptible and ≥1 μg/ml were considered non-susceptible to caspofungin. Voriconazole MICs <4 μg/ml were considered susceptible and ≥4 μg/ml were considered resistant. A subset of 62 clinical isolates were plated on chromogenic media with posaconazole added at 1-and 4-μg/ml concentrations and were also grown on control plates without drug. As breakpoints for posaconazole have not been established, as with voriconazole, MICs <4 μg/ml were considered susceptible and ≥4 μg/ml were considered resistant. Descriptive statistics were done to determine percent agreement between the two techniques (CLSI criteria vs. Chromogenic agar). For data included in these analyses, MIC values ≤0.03 mg/l were left as 0.03 and those ≥16 mg/l were left as 16 mg/l. MIC ranges (by CLSI), MIC50 and MIC90 were calculated by species. Very major errors (VME) were noted when the isolate was resistant by broth microdilution and susceptible by agar dilution. Major errors (ME) were noted when the isolate was susceptible by broth microdilution and resistant by agar dilution [15,16].
Screening mixed clinical isolates
We also evaluated a collection of 94 clinical Candida species isolates including C. albicans (22), C. glabrata (30), C. parapsilosis (19), C. tropicalis (8), C. guilliermondii (5), C. lusitaniae (2), C. rugosa (2), C. dubliniensis (1) and Candida species (5) that were obtained from the UTHSCSA Fungus Testing Laboratory. These isolates were subcultured and evaluated blindly by the agar dilution method against panels of CHROMagar plates containing anidulafungin, micafungin or caspofungin, as described above. In this set of experiments, MICs for each of the three echinocandins (anidulafungin, caspofungin, and micafungin) <1 μg/ml were considered susceptible and ≥1 μg/ml were considered non-susceptible. Descriptive statistics were done to determine percent agreement between the two techniques (CLSI criteria vs. Chromogenic agar). For these isolates, MIC data were calculated and recorded as previously noted, as were very major errors and major errors [15,16].
RESULTS
In these experiments, neither RPMI-1640 agar nor agar prepared from Antibiotic Medium 3 yielded uniform colony morphologies with the yeast species tested (data not shown). Sabouraud dextrose agar did support growth well, but produced overly luxurious growth which limited individual colony assessment which is essential for determining colony size (data not shown). In these experiments, the best overall results were observed using the chromogenic agar CHROMagar Candida, which has the added ability to guide the user to a presumptive identification of many yeast species and to quickly alert the user to mixed yeast infections. Assessment of susceptibility to the antifungal drugs was based on comparisons of colony morphology and size. Colonies grew normally on plates without antifungal drugs as shown in Figure 1, A and D. On plates with antifungal drugs, the susceptible yeasts were either significantly (<50%) reduced in size or did not grow. As shown in Figure 1-B,C,E,F, a susceptible strain of C. albicans did not grow and the resistant yeasts could be identified by color and morphology. Figure 1-B,C,E,F.
In the initial study, the effectiveness of using the agar dilution method as a screening tool for caspofungin resistance was measured using 74 clinical C. glabrata isolates of various susceptibility levels as determined by CLSI methodology with caspofungin MICs ranging from ≤0.03 to >16. Susceptibility to caspofungin was correctly assessed by agar dilution in all isolates where the CLSI caspofungin MIC was <1 μg/ml. Likewise, 18 of 20 (90%) isolates were correctly predicted to have reduced susceptibility to caspofungin, where the CLSI caspofungin MIC was ≥1 μg/ml, as shown in Figure 2-A,B,C and Table 1. Two of these isolates with elevated MICs were falsely recorded as susceptible, very major errors, as shown in Figure 3A.
Table 1.
Summary of antifungal susceptibility values determined by CLSI and agar dilution methods.
| Method |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agar Dilution | CLSI | No. of categorical errors | % Categorical error | ||||||||||
| Antifungal agent | Organism (n) | MIC min | MIC max | MIC 50 | MIC 90 | S | R* | S | R* | ME | VME | ME | VME |
| Caspofungin | C. glabrata (74) | ≤0.03 | >16 | 0.5 | 8 | 56 | 18 | 54 | 20 | 0 | 2 | 0 | 10 |
| Voriconazole | C. glabrata (76) | 0.06 | 8 | 1 | 4 | 69 | 7 | 67 | 9 | 0 | 2 | 0 | 20 |
| Posaconazole | C. glabrata (62) | ≤0.015 | >8 | 0.25 | 4 | 46 | 16 | 52 | 10 | 6 | 0 | 12 | 0 |
| Anidulafungin | Candida spp. (90) | ≤0.03 | >16 | 0.06 | 1 | 77 | 13 | 77 | 13 | 2 | 2 | 3 | 15 |
| Caspofungin | Candida spp. (94) | ≤0.03 | >16 | 0.06 | 2 | 78 | 16 | 67 | 27 | 4 | 15 | 6 | 56 |
| Micafungin | Candida spp.(91) | ≤0.03 | >16 | 0.06 | 2 | 83 | 8 | 78 | 13 | 0 | 5 | 0 | 38 |
R = notation of resistant or non-susceptible.
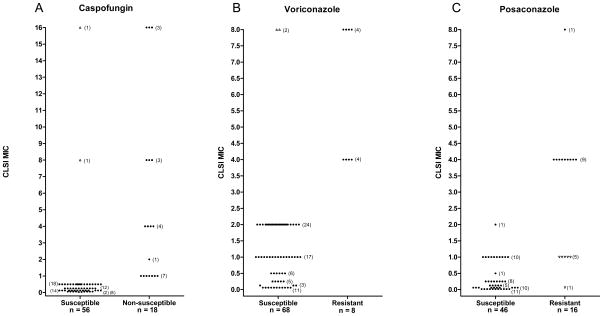
Figure 3.
Antifungal drug resistance screening of Candida glabrata comparing agar dilution to the CLSI broth microdilution method. Yeast isolates were examined on Chromagar Candida containing (A) caspofungin, (B) voriconazole or (C) posaconazole. Yeast growth on agar containing two concentrations of antifungal agent was compared to growth on drug-free agar. Yeasts were recorded as susceptible or resistant (or non-susceptible for caspofungin) on the drug screening agar and results were plotted against the CLSI MIC (plus or minus one dilution factor). Closed circles represent good correlation between the two methods. Open up triangles represent very major errors (VME) where resistant (or non-susceptible) yeasts (by CLSI) were screened as susceptible on agar dilution; open down triangles represent major errors (ME) where susceptible yeasts (by CLSI) were screened as resistant on agar dilution.
This procedure was repeated with these C. glabrata isolates using CHROMagar plates with voriconazole (MIC range 0.06 to 8). In these studies, all isolates with MIC values <4 were correctly predicted as susceptible to voriconazole (CLSI voriconazole MIC, <4 μg/ml) and 7 of 9 (78%) isolates were correctly predicted as resistant (CLSI voriconazole MIC, ≥4 μg/ml), as shown in Figure 2-D,E,F and Table 1. Very major errors were seen in two isolates that were incorrectly assessed as susceptible (Figure 3B),
A subsequent study to screen for resistance to posaconazole utilized a subset of 63 of these C. glabrata clinical isolates having CLSI MICs ranging from ≤0.015 to >8. All isolates with MIC values <4 were correctly predicted as susceptible to posaconazole (CLSI posaconazole MIC, <4 μg/ml) and all isolates with (CLSI posaconazole MIC, ≥4 μg/ml) were correctly predicted as resistant, as shown in Table 1 and Figure 3C. These studies revealed 6 major errors wherein susceptible (CLSI posaconazole MIC, <4 μg/ml) isolates (all C. glabrata) were incorrectly predicted as resistant to posaconazole (Table 1 and Figure 3C).
Despite a 22% categorical (in very major errors) error rate with voriconazole, overall, agar dilution screening of this set of C. glabrata isolates using caspofungin, posaconazole or voriconazole was shown to be 97, 97 and 90% accurate respectively in predicting antifungal resistance to those drugs compared to MICs obtained from the standard CLSI technique (Table 1).
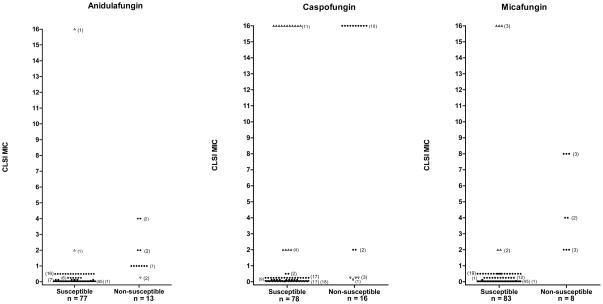
The effectiveness of using the agar dilution screening for determining the degree of echinocandin susceptibility in a more diverse group of Candida isolates was further investigated using 94 clinical Candida isolates comprised of C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. guilliermondii, C. lusitaniae, C. rugosa and C. dubliniensis, which had CLSI MICs that ranged from ≤0.03 to >16 against the three echinocandins (Tables 1 and 2). The CHROMagar technique correctly predicted anidulafungin (Table 1, Figure 4A) susceptibility for 75 of 77 (97%) isolates and non-susceptibility was correctly predicted for 11 of 13 (85%) isolates. Two very major errors and two major errors were noted comparing agar dilution to CLSI for anidulafungin. Table 1 and Figure 4B also show that caspofungin susceptibility was correctly predicted for 67 of 71 (94%) isolates (reported as 4 major errors) and non-susceptibility was correctly predicted for only 12 of 27 (45%) isolates, with a notation of 15 of 27 (55%) very major errors. Susceptibility to micafungin was correctly predicted for all CLSI susceptible isolates, however, non-susceptibility was correctly predicted for only 8 of 13 (62%) isolates, resulting in 5 very major errors as shown in Table 1 and Figure 4C. Overall, in spite of elevated percentages of very major errors (due in part to low numbers of resistant isolates), anidulafungin, caspofungin, and micafungin were shown to be 96%, 80% and 95% accurate, respectively, in predicting both susceptibility and non-susceptibility within 1 dilution factor when compared to MICs from CLSI technique.
Table 2.
Summary of antifungal susceptibility values by species determined by CLSI and agar dilution methods.
| Method |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agar Dilution | CLSI | No. of categorical errors | % Categorical error | ||||||||||
| Antifungal agent | Organism (n) | MIC min | MIC max | MIC 50 | MIC 90 | S | R* | S | R* | ME | VME | ME | VME |
| Anidulafungin | C. albicans (20) | ≤0.03 | 1 | ≤0.03 | ≤0.03 | 20 | 0 | 20 | 0 | 0 | 0 | 0 | 0 |
| Anidulafungin | C. dubliniensis (1) | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Anidulafungin | C. glabrata (29) | ≤0.03 | >16 | ≤0.03 | 1 | 23 | 6 | 23 | 6 | 1 | 1 | 4 | 17 |
| Anidulafungin | C. guilliermondii (5) | 0.125 | 1 | 0.125 | 1 | 3 | 2 | 3 | 2 | 0 | 0 | 0 | 0 |
| Anidulafungin | C. lusitaniae (2) | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Anidulafungin | C. parapsilosis (19) | 0.125 | 0.5 | 0.25 | 0.5 | 18 | 1 | 19 | 0 | 1 | 0 | 5 | 0 |
| Anidulafungin | C. rugosa (2) | ≤0.03 | 2 | ≤0.03 | 2 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 100 |
| Anidulafungin | C. tropicalis (7) | ≤0.03 | 2 | ≤0.03 | 2 | 6 | 1 | 6 | 1 | 0 | 0 | 0 | 0 |
| Anidulafungin | Candida spp. (5) | 0.125 | 4 | 1 | 4 | 2 | 3 | 2 | 3 | 0 | 0 | 0 | 0 |
| Caspofungin | C. albicans (22) | <0.03 | >16 | 0.06 | 0.125 | 21 | 1 | 21 | 1 | 0 | 0 | 0 | 0 |
| Caspofungin | C. dubliniensis (1) | <0.03 | <0.03 | <0.03 | <0.03 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Caspofungin | C. glabrata (30) | <0.03 | >16 | 0.25 | >16 | 26 | 4 | 17 | 13 | 0 | 9 | 0 | 69 |
| Caspofungin | C. guilliermondii (5) | 2 | 16 | 16 | 16 | 3 | 2 | 0 | 5 | 0 | 3 | 0 | 60 |
| Caspofungin | C. lusitaniae (2) | 0.06 | 0.125 | 0.06 | 0.125 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Caspofungin | C. parapsilosis (19) | 0.125 | 2 | 0.25 | 0.5 | 15 | 4 | 18 | 1 | 4 | 1 | 22 | 100 |
| Caspofungin | C. rugosa (2) | 16 | 16 | 16 | 16 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 100 |
| Caspofungin | C. tropicalis (8) | <0.03 | >16 | <0.03 | >16 | 7 | 1 | 7 | 1 | 0 | 0 | 0 | 0 |
| Caspofungin | Candida spp. (5) | 0.25 | >16 | 2 | >16 | 1 | 4 | 1 | 4 | 0 | 0 | 0 | 0 |
| Micafungin | C. albicans (20) | ≤0.03 | 2 | ≤0.03 | 0.25 | 19 | 1 | 19 | 1 | 0 | 0 | 0 | 0 |
| Micafungin | C. dubliniensis (1) | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Micafungin | C. glabrata (30) | ≤0.03 | >16 | ≤0.03 | 4 | 26 | 4 | 24 | 6 | 0 | 2 | 0 | 33 |
| Micafungin | C. guilliermondii (5) | 0.125 | 0.5 | 0.25 | 0.5 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| Micafungin | C. lusitaniae (2) | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Micafungin | C. parapsilosis (19) | 0.25 | 0.5 | 0.5 | 0.5 | 19 | 0 | 19 | 0 | 0 | 0 | 0 | 0 |
| Micafungin | C. rugosa (2) | ≤0.03 | 16 | ≤0.03 | 16 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 100 |
| Micafungin | C. tropicalis (7) | ≤0.03 | 16 | ≤0.03 | 16 | 7 | 0 | 6 | 1 | 0 | 1 | 0 | 100 |
| Micafungin | Candida spp. (5) | 0.5 | 8 | 2 | 8 | 2 | 3 | 1 | 4 | 0 | 1 | 0 | 25 |
R = notation of resistant or non-susceptible.
Figure 4.
Antifungal drug resistance screening of clinical Candida isolates comparing agar dilution to the CLSI broth microdilution method. Yeast isolates were examined on Chromagar Candida containing (A) anidulafungin, (B) caspofungin or (C) micafungin. Yeast growth on agar containing two concentrations of antifungal agent was compared to growth on drug-free agar. Yeasts were recorded as susceptible or resistant (or non-susceptible for the echinocandins) on the drug screening agar and results were plotted against the CLSI MIC (plus or minus one dilution factor). Closed circles represent good correlation between the two methods. Open up triangles represent very major errors (VME) where resistant yeasts (by CLSI) were screened as susceptible on agar dilution; open down triangles represent major errors (ME) where susceptible yeasts (by CLSI) were screened as resistant on agar dilution.
The results of overall screening of individual species for susceptibility to the three echinocandins tested are presented in Table 2. Determinations of echinocandin susceptibility in C. albicans, compared very favorably to the CLSI MIC values although few isolates with resistant isolates were tested. All isolates were correctly determined to be susceptible or non-susceptible to anidulafungin, caspofungin and micafungin. Furthermore, agar dilution screening of C. tropicalis performed well, where only 1 error was noted against the three echinocandins: 1 very major error was seen when testing against micafungin.
Examination of the agar dilution susceptibility data for.C. glabrata reveals an increase in the number of errors in comparison to CLSI. With anidulafungin tested against C. glabrata, we noted 1 major error (1 of 23, 4%) and 1 very major error (1 of 6, 17%). With caspofungin, we noted 9 very major errors (9 of 13, 69%), but no major errors, and in the case of micafungin, we recorded 2 very major errors (2 of 6, 33%) and no major errors.
In addition, elevated error rates were seen in the agar dilution assessment of susceptibility against caspofungin in C. parapsilosis clinical isolates, wherein 4 major errors (4 of 18, 22%) and 1 very major error (100%) were recorded. Nevertheless, there were no errors noted comparing agar dilution screening of C. parapsilosis susceptibility to CLSI, and only one major error (1 of 18, 6%) was seen with the use of anidulafungin agar dilution screening.
Finally, as shown in Table 2, we examined agar dilution screening of echinocandin susceptibility against small numbers of C. guilliermondii, C. rugosa and Candida spp. isolates. In the case of C. guilliermondii (in which all 5 isolates were non-susceptible to caspofungin), 3 very major errors were noted (3 of 5, 60%) in testing caspofungin by agar dilution, however, there were no errors in the assessments of anidulafungin or micafungin. Assessments of C. rugosa by agar dilution revealed the most errors, percentagewise, by species for the three echinocandins. With anidulafungin and micafungin, there was 1 very major error (1 of 2, 50%) for each drug and with caspofungin, there were 2 very major errors (2 of 2, 100%) noted as compared to the CLSI results.
DISCUSSION
In previous studies investigating resistance to fluconazole in Candida, we utilized CHROMagar Candida containing fluconazole at 8 and 16 μg/ml and compared yeast growth on these plates to growth on control plates without drug [4,5]. In the current studies, we desired to investigate the utility of agar dilution screening of Candida for resistance to the newer azoles and echinocandins. Breakpoints for voriconazole have been recently established showing susceptibility at <1 μg/ml and resistance at ≥4 μg/ml of drug concentrations [1,7,17,18]. Therefore, we chose to investigate both voriconazole and posaconazole at these levels. At the time these studies were initiated, there were no established breakpoints for the echinocandins [18]. Pilot studies in our laboratory showed that echinocandin drug concentrations of 0.5 and 1 μg/ml were useful for our screening purposes, and studies were designed using those antifungal levels in the medium. Recently, echinocandin breakpoints have been published with MICs ≤2 being noted as susceptible and MICs >2 being noted as non-susceptible, rather than “resistant” since in the absence of clinical studies, the significance of decreased susceptibility is not demonstrated for this class of drugs [1].
The choice of growth medium affects effectiveness of agar dilution as a screening tool for antifungal resistance. We demonstrated this in our previous studies with fluconazole [4,5] and have found that the same concept holds true with the two azoles and the three echinocandins that we evaluated in this study. In these studies, two choices for the base medium, namely RPMI-1640 and Antibiotic Medium 3, failed to support adequate and uniform yeast growth and were eliminated from further study for that reason. Both Sabouraud dextrose agar and CHROMagar Candida supported growth of the yeasts that we used, yet we found that growth on Sabouraud dextrose agar was overly abundant. The uniform and consistent pattern of yeast growth obtained with CHROMagar Candida, combined with its chromogenic properties makes it an ideal medium to use as the basis for agar dilution screening. In addition, CHROMagar Candida allows early detection of mixed yeast populations [4,5].
We have previously shown that the use of agar dilution screening yeasts demonstrating resistance to fluconazole was useful in oral rinse samples [19,20] as well as in purified cultures [4,5]. In our current examination of the utility of agar dilution screening of C. glabrata for antifungal resistance, we found that the addition of caspofungin, posaconazole or voriconazole to CHROMagar Candida allowed effective, early prediction of antifungal susceptibility to these drugs. With these three drugs, our ability to accurately and rapidly screen antifungal susceptibility in C. glabrata exceeded 90% in comparison to the established CLSI methodology. All three drugs showed excellent agreement between the two methods in determining susceptible isolates, with only 6 major errors where susceptible isolates were predicted to be resistant by agar dilution on posaconazole plates and 4 very major errors, two each for caspofungin and voriconazole, wherein CLSI resistant/non-susceptible isolates were determined to be susceptible by agar dilution Most of the discrepancies in resistance screening of C. glabrata alone occurred with posaconazole, where 6 of 52 (12%) susceptible isolates were falsely determined to be resistant. Nevertheless, overall agreement between agar dilution screening for reduced susceptibility of C. glabrata to caspofungin, posaconazole and voriconazole as compared to the CLSI standard was 97, 90 and 97% accurate, respectively, which correlates well with our previously published fluconazole-resistance screening results for Candida.
Similarly, we found a high degree of correlation between the standard CLSI susceptibility testing and agar dilution screening of Candida resistance to the echinocandins, anidulafungin, caspofungin and micafungin against a more diverse collection of Candida including C. guilliermondii and C. parapsilosis. As noted previously, these two organisms were examined in order to provide a more stringent assessment of the screening method. These yeasts are more likely to show reduced susceptibility to the echinocandins than would C. albicans or C. tropicalis although in our series, C. parapsilosis isolates screened were susceptible to echinocandins by CLSI methods. We found that accurate determination of yeast susceptibility by agar dilution with two of the three echinocandins was very high, at over 95% for anidulafungin and micafungin. As previously noted, though, there were more discrepancies in assessing the non-susceptible yeasts in our expanded panel of Candida isolates. Interestingly, in the extended panel of Candida species we noted a higher incidence of very major errors (false assessment of susceptibility), which was particularly prevalent among the C. glabrata isolates during screening against caspofungin. This difference, may, in part, be due to the relatively high percentage of non-susceptible isolates in that panel. In our initial studies of C. glabrata, the MIC90 was 8, and there were 20 of 74 (27%) isolates with reduced susceptibility to caspofungin. In contrast, in the second set of studies, the MIC90 for the C. glabrata isolates (against caspofungin) was >16 and there were 13 of 30 (43%) isolates with reduced susceptibility to caspofungin, although clinically resistance of C. glabrata remains uncommon [21]
In these studies, we found that the inclusion of C. parapsilosis made the assessment more difficult. In these studies, C. parapsilosis was a difficult organism to evaluate. In many cases, these isolates did not grow heavily on the control or drug-test plates, which confounded the assessment of resistant (or non-susceptible) organisms, which was reflected in the discrepancies detected for that species. Overall, major errors or very major errors for the echinocandins for this organism ranged from 0 to 22% and 0 to 100%, respectively.
Lastly, we found that in the three assessments of echinocandins against over 90 varied Candida isolates, we noted a total of 6 major errors and 22 very major errors. In examining the individual data, we found that 3 different C. glabrata, 1 C. parapsilosis and 1 C. rugosa isolates were responsible for half (11 of 22) of the very major errors in this series of experiments.
One of the limitations to this study was the small number of resistant/non-susceptible isolates that were evaluated. The diverse group of clinical isolates under study against the three echinocandins was included as a more stringent test of the method. Indeed, in comparing the percentages of major errors and very major errors within those studies we found that the agar dilution screening method for the echinocandins was effective in determining the susceptible isolates, with more errors noted in determining the non-susceptible isolates. Agar dilution susceptibility screening provided an accurate measure of susceptibility of both the echinocandins and newer azoles in this study.
There are certain difficulties related to the assessment of resistance based on colony size. Accuracy in visually assessing reduced colony size on media with antifungal drugs, that is, ≤50% size, as compared to growth on drug-free media, may be influenced in several ways. We have noted growth differences by individual organism, by medium, and by antifungal drug.
In conclusion, we found that there are differences in the ability of growth media to support agar dilution screening of Candida yeasts for antifungal resistance. In these studies, CHROMagar Candida was a superior medium for this purpose. These studies demonstrated excellent correlation with the CLSI method in determining antifungal drug susceptibility to anidulafungin, micafungin, posaconazole and voriconazole against clinical Candida species. The utility of agar dilution screening with the echinocandins, particularly against a more extensive collection of isolates with reduced susceptibility, warrants further investigation.
Acknowledgments
We are indebted to A.C. Vallor and M.C. Olivo, Jr. for their technical expertise in carrying out these studies.
FUNDING
This project was funded in part by federal funds from the National Institute of Dental and Craniofacial Research, National Institutes of Health, under Contract No. NIH NIDCR 5R01-DE018096 and NIH Grant #DE14318 for the COSTAR Program
Footnotes
TRANSPARENCY DECLARATIONS
WRK, JDZ, FPH, MJB, EB, AWF, DIM: None to declare
TFP: Research Support: Merck & Co., Pfizer Inc., and Schering-Plough Corporation, Speakers Bureau: Merck & Co., Pfizer Inc.; Consultant: Basilea, Merck & Co., Pfizer Inc., Schering- Plough Corporation, and Toyoma.
SWR: Research Support: Pfizer Inc., Schering-Plough Corporation, Astellas Pharma US Inc.
References
- 1.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute; Villanova, PA: 2008. [Google Scholar]
- 2.Alexander BD, Byrne TC, Smith KL, et al. Comparative evaluation of Etest and sensititre yeastone panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol. 2007;45:698–706. doi: 10.1128/JCM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diekema DJ, Messer SA, Hollis RJ, et al. Evaluation of Etest and disk diffusion methods compared with broth microdilution antifungal susceptibility testing of clinical isolates of Candida spp. against posaconazole. J Clin Microbiol. 2007;45:1974–1977. doi: 10.1128/JCM.02087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson TF, Kirkpatrick WR, Revankar SG, et al. Comparative evaluation of macrodilution and chromogenic agar screening for fluconazole susceptibility of Candida albicans. J Clin Microbiol. 1996;34:3237–3239. doi: 10.1128/jcm.34.12.3237-3239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson TF, Revankar SG, Kirkpatrick WR, et al. Simple method for detecting fluconazole-resistant yeasts with chromogenic agar. J Clin Microbiol. 1996;34:1794–7. doi: 10.1128/jcm.34.7.1794-1797.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfaller MA, Boyken L, Hollis RJ, et al. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp. including 315 isolates resistant to fluconazole. J Clin Microbiol. 2005;43:5425–5427. doi: 10.1128/JCM.43.11.5425-5427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller MA, Diekema DJ, Rex JH, et al. Correlation of MIC with outcome for Candida species tested against voriconazole: analysis and proposal for interpretive breakpoints. J Clin Microb. 2006;44:819–826. doi: 10.1128/JCM.44.3.819-826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J Clin Microbiol. 2008;46:150–156. doi: 10.1128/JCM.01901-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redding SW, Marr KA, Kirkpatrick WR, et al. Candida glabrata sepsis secondary to oral colonization in bone marrow transplantation. Med Mycol. 2004;42:479–481. doi: 10.1080/13693780410001731574. [DOI] [PubMed] [Google Scholar]
- 10.Redding SW, Zellars RC, Kirkpatrick WR, et al. Epidemiology of oropharyngeal Candida colonization and infection in patients receiving radiation for head and neck cancer. J Clin Microbiol. 1999;37:3896–3900. doi: 10.1128/jcm.37.12.3896-3900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barchiesi F, Spreghini E, Tomassetti S, et al. Comparison of the fungicidal activities of caspofungin and amphotericin B against Candida glabrata. Antimicrob Agents Chemother. 2005;49:4989–4992. doi: 10.1128/AAC.49.12.4989-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbrecht R, Nivoix Y, Fohrer C, et al. Management of systemic fungal infections: alternatives to itraconazole. J Antimicrob Chemother. 2005;56 (Suppl 1):i39–i48. doi: 10.1093/jac/dki223. [DOI] [PubMed] [Google Scholar]
- 13.Zaoutis TE, Foraker E, McGowan KL, et al. Antifungal susceptibility of Candida spp. isolated from pediatric patients: a survey of 4 children’s hospitals. Diagn Microbiol Infect Dis. 2005;52:295–298. doi: 10.1016/j.diagmicrobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Brockway EB, Coco J, Kirkpatrick WR, et al. Screening for Candida glabrata susceptibility using CHROMagar Candida containing fluconazole. Abstr. C-135. P. 120. Abstr. 102nd Gen. Meet. Am. Soc. Microbiol; 2002; Salt Lake City, UT. [Google Scholar]
- 15.Cantón E, Pemán J, Espinel-Ingroff A, et al. Comparison of disc diffusion assay with the CLSI reference method (M27-A2) for testing in vitro posaconazole activity against common and uncommon yeasts. J Antimicrob Chemother. 2008;61:135–138. doi: 10.1093/jac/dkm442. [DOI] [PubMed] [Google Scholar]
- 16.Espinel-Ingroff A, Cantón E. Comparison of Neo-Sensitabs Tablet Diffusion Assay with CLSI Broth Microdilution M38-A and Disk Diffusion Methods for Testing Susceptibility of Filamentous Fungi with Amphotericin B, Caspofungin, Itraconazole, Posaconazole, and Voriconazole. J Clin Microbiol. 2008;46:1793–1803. doi: 10.1128/JCM.01883-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arikan S. Current status of antifungal susceptibility testing methods. Med Mycol. 2007;45:569–587. doi: 10.1080/13693780701436794. [DOI] [PubMed] [Google Scholar]
- 18.Dannaoui E, Lortholary O, Raoux D, et al. Comparative in vitro activities of caspofungin and micafungin, determined using the method of the European Committee on Antimicrobial Susceptibility Testing, against yeast isolates obtained in France in 2005–2006. Antimicrob Agents Chemother. 2008;52:778–781. doi: 10.1128/AAC.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redding SW, Dahiya MC, Kirkpatrick WR, et al. Candida glabrata is an emerging cause of oropharyngeal candidiasis in patients receiving radiation for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:47–52. doi: 10.1016/j.tripleo.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Westbrook SD, Kirkpatrick WR, Freytes CO, et al. Candida krusei sepsis secondary to oral colonization in a hemopoietic stem cell transplant recipient. Med Mycol. 2007;45:187–190. doi: 10.1080/13693780601164306. [DOI] [PubMed] [Google Scholar]
- 21.Thompson GR, 3rd, Wiederhold NP, Vallor AC, et al. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob Agents Chemother. 2008;52:3783–3785. doi: 10.1128/AAC.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]