Table 2.
Synthetic potential of trisubstituted ureas as masked isocyanates.
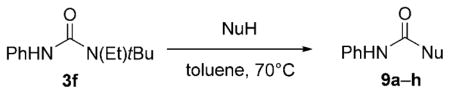 | ||||
|---|---|---|---|---|
| Entry | NuH (equiv) | t [h] | Product | Yield [%][a] |
| 1 | H2O[b] | 1 | 9 a[c] | >99 |
| 2 | MeOH (1.1) | 6 | 9 b | >99 |
| 3 | tBuOH[b,d] | 8 | 9 c | 70 |
| 4 | PhCH2OH (1.1) | 8 | 9 d | 94 |
| 5 | 9-fluorenylmethanol (2) | 5 | 9 e | 78 |
| 6 | PhOH (2) | 18 | 9 f | 72 |
| 7 | PhSH (2) | 18 | 9 g | 62 |
| 8 | tBuNH2 (1.1) | 5 | 9 h | >99 |
Yield of isolated product.
Used as a solvent.
The aniline generated through decarboxylation is trapped in situ to yield 1,3-diphenylurea.
Reaction was performed at 90 °C. Nu =nucleophile.
