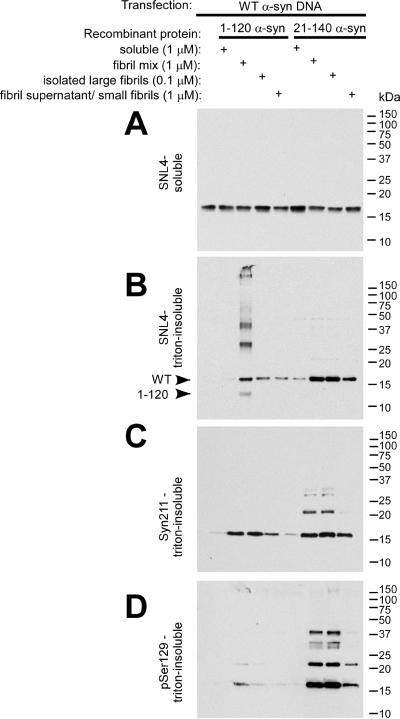
Figure 3.
Biochemical cellular fractionation of QBI293 cells after transfection and seeding with recombinant α-syn fibrils. QBI293 cells were transfected with the WT α-syn expression plasmid. After transfection, recombinant 1–120 α-syn or 21–140 α-syn was added to the media. The recombinant protein was added in soluble form, after fibrillization in vitro (fibril mix), or after fibrillization and isolation by centrifugation at 16,000 × g, where the pellet (large fibrils) was isolated from the supernatant (small fibrils/polymers/soluble protein). Western blot analysis of α-syn immunoreactivity was performed after biochemical cellular fractionation isolating soluble from triton-insoluble α-syn, as described in “Materials and Methods.” The first lane at the left shows biochemical fractionation of cells that did not receive any recombinant α-syn treatment. (A) SNL4 immunoreactivity of soluble samples recognized soluble WT α-syn, resulting from cellular transfection. Triton-insoluble protein was analyzed with (B) SNL4, (C) Syn211, and (D) pSer129 antibodies, and α-syn was identified in samples treated with recombinant fibrillized α-syn protein. Migration of α-syn protein and epitope-specific antibodies show that the addition of recombinant fibrillized protein induced aggregation of endogenously expressed α-syn. Arrowheads indicate the immunobands corresponding to full-length WT α-syn (WT) and 1–120 α-syn (1–120).