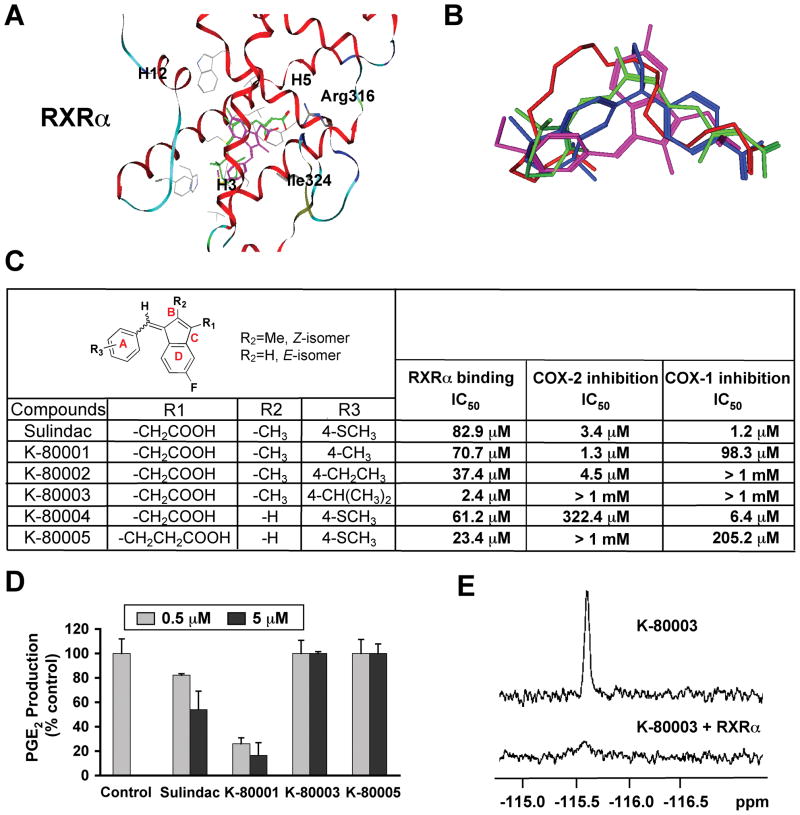
Figure 7. Design, Synthesis and Evaluation of RXRα-selective Sulindac Analogs.
(A) Docking of sulindac sulfide (magenta) to the LBP of RXRα in reference to 9-cis-RA (green). Side chains within 4Å of the ligands are displayed in grey.
(B) Comparison of orientation and position of docked sulindac sulfide (magenta) to the crystal structures of 9-cis-RA (green), DHA (red) and BMS649 (blue).
(C) RXRα binding and inhibition of COX-1 and COX-2 activities by Sulindac analogs. RXRα binding was measured by competition ligand-binding assays. COX inhibition assays used Cayman’s COX Fluorescent Activity Assay Kits.
(D) Inhibition of PGE2 production by Sulindac and analogs. A549 cells stimulated with TNFα (10 ng/ml) for 24 hr were treated with Sulindac or analogs for 30 min. PGE2 production was measured and expressed as the ratio of PGE2 produced in the presence of compound to that with vehicle. Error bars represent SEM.
(E) Comparison of 19F NMR spectra of K-80003 (100 μM) in the absence and presence of 10 μM RXRα LBD.
One of three to five similar experiments is shown.
See also Figure S7.