Fig. 2.
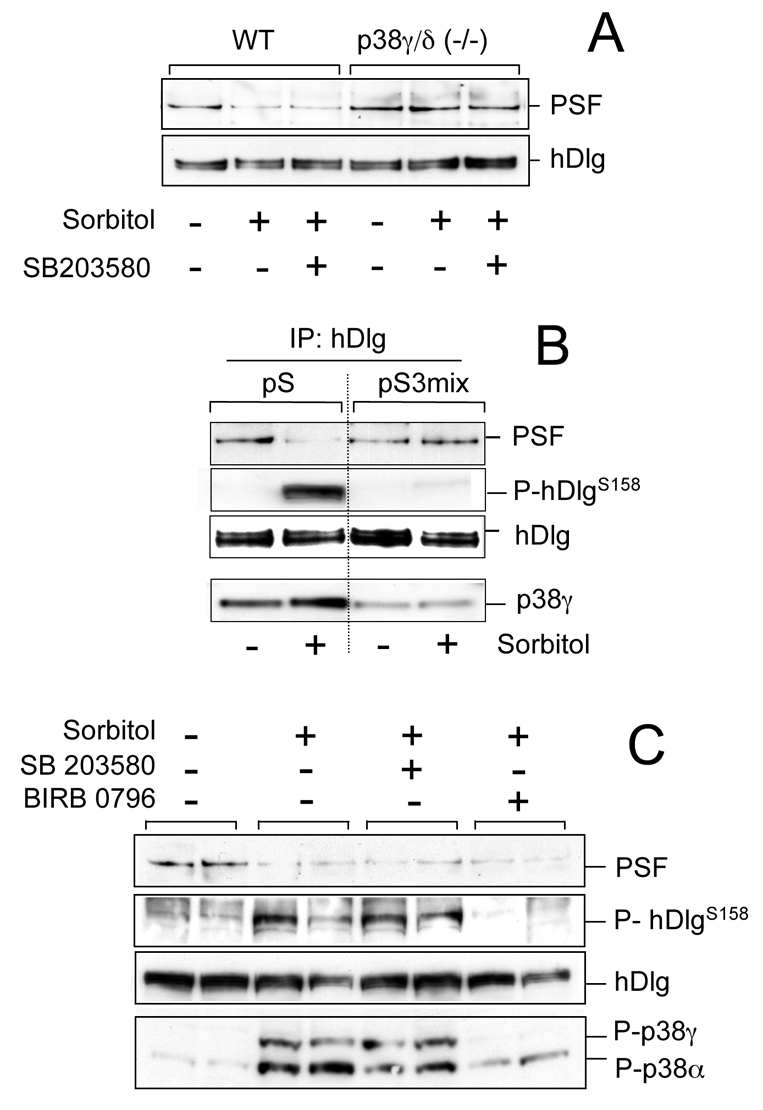
p38γ phosphorylation of hDlg does not regulate its interaction with PSF. (A) WT or p38γ/δ−/− MEFs were incubated (1 hour) alone or with 10 μM SB203580, then exposed to 0.5 M sorbitol (15 minutes). Endogenous hDlg was immunoprecipitated with anti-hDlg antibody and pellets immunoblotted with anti-PSF or anti-hDlg antibody. (B) HEK293 cells were transfected with empty pSuper vector (pS) or with a mixture of siRNA constructs to knock down p38γ (pS3mix). Cells were treated as in A, endogenous hDlg was immunoprecipitated from 0.5 mg cell lysates and pellets were immunoblotted with anti-PSF, anti-P-hDlgS158 or anti-hDlg antibody. Total lysates were immunoblotted with an antibody against total p38γ. (C) HEK293 cells were incubated alone or with 10 μM SB203580 or 1 μM BIRB 0796 (1 hour), then exposed to 0.5 M sorbitol (15 minutes). Endogenous hDlg was immunoprecipitated with anti-hDlg antibody and pellets were immunoblotted as in B. Total lysates were immunoblotted with an antibody that recognises phosphorylated p38γ and p38α.
