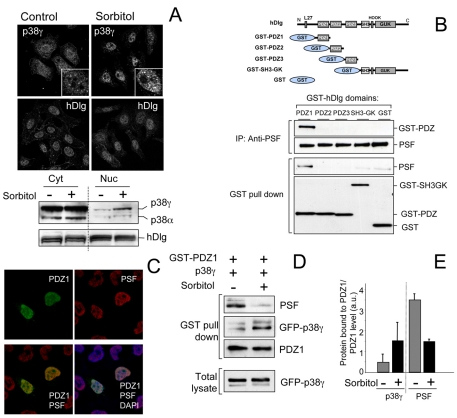
Fig. 5.
p38γ accumulates in the nucleus after osmotic stress and regulates hDlg-PSF interaction. (A) HeLa cells, unstimulated or treated with 0.5 M sorbitol (15 minutes), were stained with anti-p38γ or anti-hDlg antibodies and analysed by fluorescence microscopy. Inserts in the top panels show a higher magnification of p38γ localization in cell nuclei. Cells were fractionated and 30 μg protein from cytoplasm (Cyt) and nuclear (Nuc) fractions were immunoblotted using anti-p38γ, anti-p38α and anti-hDlg antibodies. (B) Different hDlg domains were expressed in HEK293 cells (top). After transfection, cells were lysed and GST-proteins purified by pull-down. Endogenous PSF was immunoprecipitated using anti-PSF antibody. Pellets were immunoblotted using anti-PSF or anti-GST antibody. (C) HEK293 cells were transfected with GST-PDZ1, stained with anti-GST and/or anti-PSF antibody, and analysed by fluorescence microscopy. Nuclei are stained with DAPI. Results were similar in four independent experiments in HEK293 cells and in two independent experiments in HeLa cells. (D) HEK293 cells expressing GST-PDZ1 and GFP-p38γ were treated with sorbitol as in A. GST-PDZ1 was purified by pull-down immunoprecipitation and pellets were immunoblotted using anti-PSF, anti-GST or anti-GFP antibodies. Total lysates were immunoblotted with anti-GFP antibody to examine p38γ expression. (E) Band intensity of blots in D (anti-PSF, anti-GST for PDZ1 and anti-GFP for p38γ) were quantified and the relative amount of p38γ or PSF in pellets was calculated. Histogram values are means ± s.e.m. of four independent experiments.