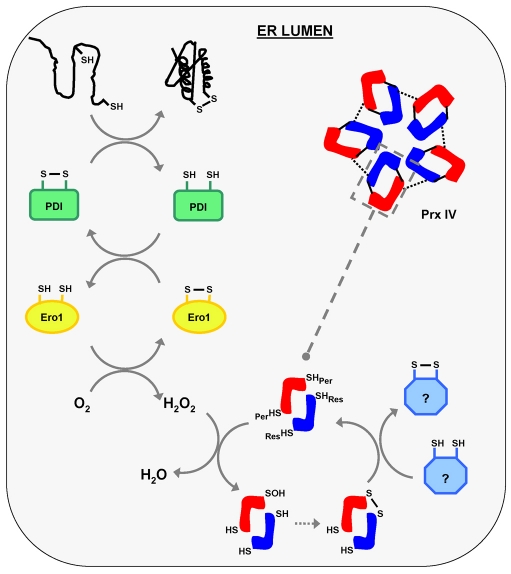
Fig. 6.
PrxIV metabolises hydrogen peroxide produced during formation of disulphide bonds in the human ER. Schematic illustrating the role of PrxIV in peroxide elimination following oxidation of PDI by Ero1. Oxidation of PDI facilitates the introduction of a disulphide into a client protein during PDI-catalysed oxidative folding (depicted top left). As Ero1 is re-oxidised by molecular oxygen the hydrogen peroxide produced is catabolised by PrxIV, resulting in changes in PrxIV redox state (SHPer, peroxidatic cysteine; SHRes, resolving cysteine). Continued peroxidase activity requires a thiol-dependent recycling pathway (represented in light blue on bottom right) which clearly exists for PrxIV. This might involve a thioredoxin-domain-containing PDI family member, but its nature remains unclear.