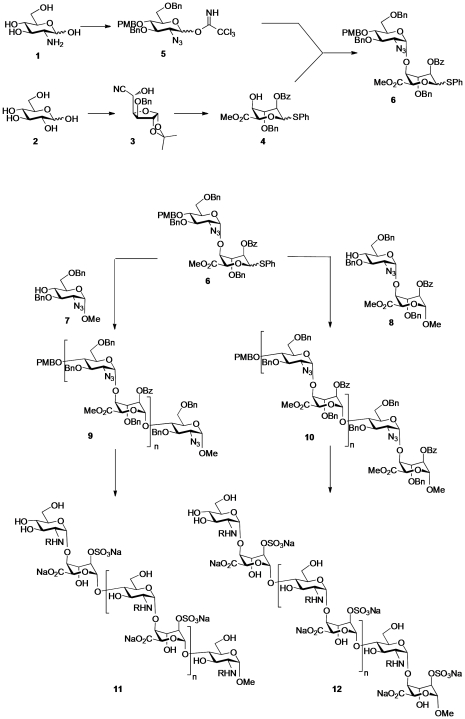
Figure 1. Strategy for assembly of odd- and even-numbered oligosaccharides.
D-Glucose was converted to the L-ido cyanohydrin 3 and further modifications gave the L-iduronic acid acceptor 4. This acceptor was coupled with the donor 5, derived from D-glucosamine 1, to yield the disaccharide donor 6 containing a reactive thioglycoside unit. The donor disaccharide 6 was elongated by reaction with either acceptor monoglucoside 7 or with acceptor disaccharide 8. The resulting tri- and tetrasaccharides 9 and 10, were further homologated by iterative cycles consisting of a 2-step process of selective removal of PMB group followed by addition of another disaccharide donor unit, 6. The final odd- and even-numbered oligomers 9 and 10, respectively, were then deprotected by sequential ester hydrolysis to allow 2-O-sulfation, followed by hydrogenolysis to remove benzylic ethers and reduce azido groups and finally an optional selective N-sulfation to yield the oligomers 11 and 12. 11: 7-mer 2S: n = 2, R = -H; 7-mer 2SNS: n = 2, R = -SO3Na; 9-mer 2S: n = 3, R = -H; and 9-mer 2SNS: n = 3, R = -SO3Na. 12: 8-mer 2S: n = 2, R = -H; 8-mer 2SNS: n = 2, R = -SO3Na; 10-mer 2S: n = 3, R = -H; 10-mer 2SNS: n = 3, R = -SO3Na; 12-mer 2S: n = 4, R = -H; and 12-mer 2SNS: n = 4, R = -SO3Na.