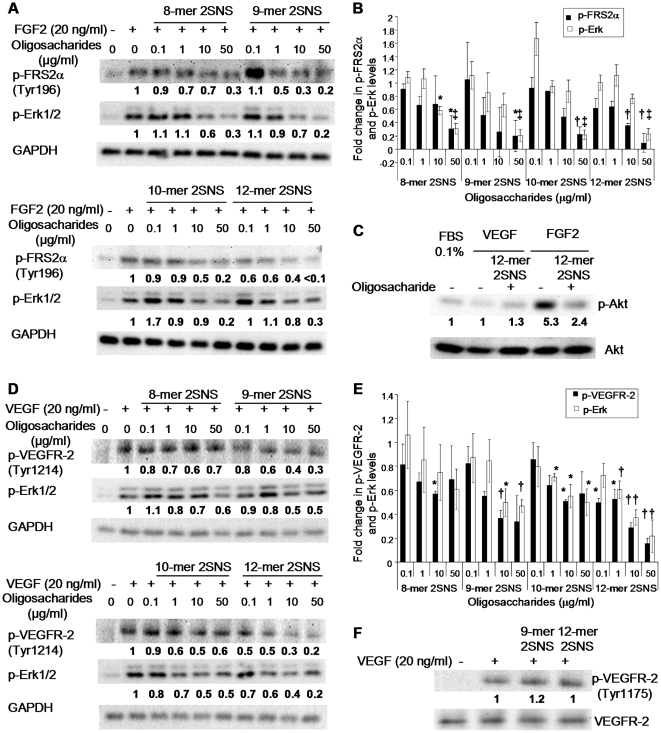
Figure 7. Oligosaccharides inhibit FGF2- and VEGF165-induced receptor activation and signalling.
(A) Serum-starved HUVECs were stimulated with FGF2 for 10 min in the absence or presence of increasing concentrations of indicated oligosaccharides. Phosphorylated FRS2 and Erk were detected by Western blotting. Equal protein loading was monitored by probing with the anti-GAPDH antibody. The values below each blot represent an average normalized fold change in the intensities of bands as compared to the bands corresponding to FGF2 stimulated cells in the absence of oligosaccharides. The normalized fold change was derived from three independent experiments. (B) Fold change of phosphorylated FRS2 and Erk levels in FGF2 treated cells in the presence of oligosaccharides as compared to the FGF2 stimulated cells without oligosaccharide treatment. Amount of phosphorylated FRS2 and Erk as determined by densitometric evaluation was normalized against corresponding GAPDH levels. Values are the mean ± SEM (n = 3). *, P<0.05; †, P<0.01; ‡, P<0.0001. (C) Biologically active 12-mer 2SNS inhibits FGF2-induced activation of PI3K/Akt pathway. Serum-starved HUVECs were exposed to FGF2 (20 ng/ml) or VEGF165 (20 ng/ml) for 10 min in the absence or presence of 12-mer 2SNS (50 µg/ml; 14.7 µM). Phospho-Akt (Ser473) was detected by immunoblotting with the anti-phospho-Akt antibody recognising Akt phosphorylation on serine 473. Total Akt protein levels were assessed by probing with the anti-Akt antibody. Average fold change values derived from three independent experiments show no stimulation of Akt phosphorylation by VEGF165 and inhibition of FGF2-induced phosphorylation of Akt by 12-mer 2SNS. (D) VEGF165 stimulation was performed in the absence or presence of increasing concentrations of respective oligosaccharides. Phosphorylation of VEGFR-2 on tyrosine 1214 and phospho-Erk were detected by immunoblotting with the respective antibodies as shown. GAPDH levels show the total protein levels. Average fold change derived from three independent experiments shows significant activity of 12-mer 2SNS in inhibiting phosphorylation of VEGFR-2 and Erk. (E) Fold change of phosphorylated VEGFR-2 (Tyr1214) and Erk levels in VEGF165 stimulated cells in the presence of oligosaccharides as compared to VEGF165 stimulated cells without oligosaccharide treatment. The amount of phosphorylated proteins was normalized against corresponding GAPDH levels. Values are the mean ± SEM (n = 3). *, P<0.05; † P<0.01. (F) 12-mer 2SNS is not affecting phosphorylation of VEGFR-2 on tyrosine 1175. VEGF165 treatment was performed for 5 min in the absence or presence of 9-mer 2SNS or 12-mer 2SNS dosed at 50 µg/ml (9-mer 2SNS 19.6 µM and 12-mer 2SNS 14.7 µM) concentration. Phosphorylation of VEGFR-2 on tyrosine 1175 was detected by probing with the indicated antibody. Anti-VEGFR-2 antibody was used to determine the total VEGFR-2 protein levels. Densitometric quantification of three independent blots shows no effect on phospho-VEGFR-2 (Tyr 1175) levels.