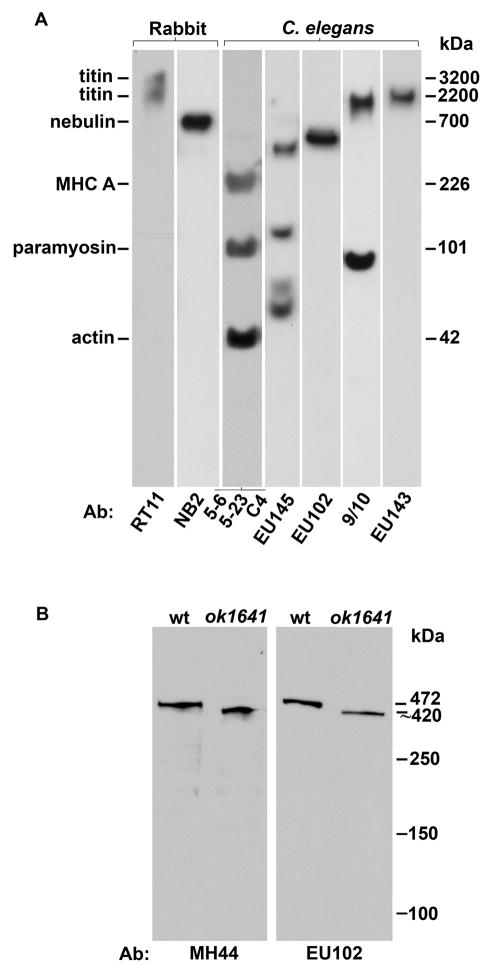
Fig. 2. Identification of a 2.2 MDa TTN-1 by immunoblot and site-specific antibodies.
(A) TTN-1 specific antibodies: Total proteins from a mixed-stage population of C. elegans (Ce), or rabbit myofibril proteins (Rb) were separated on a 2–8% gradient gel and transferred to PVDF membrane. Individual strips were incubated with either antibodies to vertebrate titin (3.2 and 2.2. MDa) or nebulin (700 kDa) or a mixture of antibodies to nematode myosin heavy chain (MHC, 200 kDa), paramyosin (90 kDa) or actin (43 kDa) to serve as size markers. Of the four antibodies produced to different regions of TTN-1(EU145, EU102, 9/10 and EU143), only 9/10 and EU143 detect a polypeptide from worm extracts running at approx. 2 MDa, the size predicted for TTN-1 from previous sequence analysis.16
(B) Kettin-specific antibody: Total proteins from either wild type (wt) or a strain carrying an intragenic deletion in the kettin gene (ok1641) were separated on a 5% gel, transferred to membrane and reacted with either EU102 or MH44, an monoclonal shown previously to recognize kettin.18 As shown, both antibodies recognize a protein of approx. 500 kDa, the size of kettin, from wild type and detect a slightly smaller protein in ok1641. Thus, although EU102 was generated to TTN-1 sequence, it clearly detects a different protein, kettin and displayed no reactivity with TTN-1 on immunoblot.