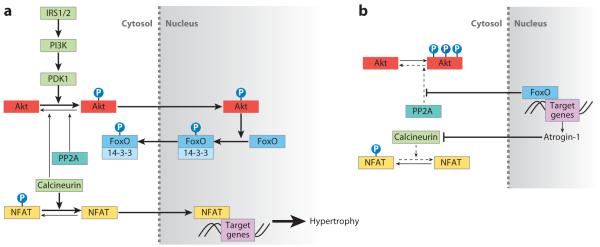
Figure 1.
Akt and FoxO signaling pathways in cardiac hypertrophy. (a) Insulin receptor signaling activates the PI3K (phosphoinositide-3 kinase) signaling cascade, leading to Akt phosphorylation and translocation into the nucleus. Akt phosphorylates three conserved FoxO residues to promote FoxO association with 14-3-3 proteins and nuclear export to the cytosol, where the complex remains transcriptionally inactive. Calcineurin dephosphorylates NFAT (nuclear factor of activated T cells), allowing NFAT nuclear translocation and subsequent transcription of NFAT target genes, such as those encoding α-skeletal actin and β-myosin heavy chain. PP2A and calcineurin provide negative feedback by dephosphorylating Akt. (b) In the absence of PI3K signaling, FoxO is able to transcribe target genes such as atrogin-1, which encodes a ubiquitin ligase that promotes calcineurin degradation and prevents NFAT activation. The decrease in calcineurin levels reduces Akt-associated phosphatase activity. FoxO also reduces PP2A phosphatase activity on Akt. The reduction in calcineurin and PP2A-associated phosphatase activity leads to Akt hyperphosphorylation, which eventually inhibits FoxO activity.