Abstract
Background
Although recent reviews have suggested active smoking to be a risk factor for breast cancer, the association with passive smoke exposure remains controversial. This risk association was explored in a large prospective study of women, the California Teachers Study.
Methods
Detailed lifetime information on passive smoke exposure by setting (home, work, or social) and by age of exposure were collected in 1997 from 57,523 women who were lifetime nonsmokers and had no history of breast cancer. In the ensuing decade, a total of 1,754 women were diagnosed with invasive breast cancer. Cox proportional hazards models were fit to estimate hazard ratios (HRs) and 95% confidence intervals (95% CI) associated with several lifetime passive smoke exposure metrics.
Results
For all breast cancer, measures of higher lifetime passive smoking intensity and duration were associated with non-statistically significant HRs of 1.11 to 1.14. For postmenopausal women, HRs for lifetime low, medium and high cumulative exposure were 1.17 (95%CI 0.91, 1.49), 1.19 (95%CI 0.93, 1.53), and 1.26 (95% CI 0.99, 1.60). For women exposed in adulthood (age ≥20) risk was elevated at the highest level of cumulative exposure (HR=1.18, 95% CI 1.00, 1.40), primarily among postmenopausal women (HR=1.25, 95% CI 1.01, 1.56). A statistically significant dose response was detected when analysis was restricted to women with moderate to high levels of passive smoke exposure.
Conclusion
These results suggest that cumulative exposures to high levels of side stream smoke may increase breast cancer risk among postmenopausal women who themselves have never smoked tobacco products.
Keywords: passive smoking, tobacco, breast cancer, cohort study, women
Introduction
It is widely acknowledged that tobacco smoke is a human carcinogen (1). Approximately 60 percent of nonsmokers in the U.S. show biological evidence of exposure (2). Cumulating evidence, most recently reviewed by a panel on tobacco smoke and breast cancer risk commissioned by the Ontario Tobacco Research Unit and the Public Health Agency of Canada (3), has implicated active smoking as a contributor to women's risk of breast cancer. The evidence for a relationship between passive smoking and breast cancer, however, remains more tenuous. Two of the key research recommendations from the 2009 Canadian report called for studies with comprehensive measures of lifetime exposure to tobacco smoke and measures of exposure at targeted periods of potentially increased susceptibility.
The California Teachers Study (CTS), a large ongoing prospective study of women, is particularly well suited to address those issues. An initial report on active and passive smoking risks for breast cancer in the CTS found significantly higher risks for subsequently developing breast cancer among women who were current smokers at the time they completed a baseline questionnaire (4). No such risks were noted for passive smoke exposures, which were limited to information about household sources of exposure in that same baseline questionnaire. This study provides the foundation to explore these risk relationships in a more substantive way because we queried respondents in a second questionnaire to obtain a full characterization of passive smoke exposure across time periods, ages of exposure and settings (home, workplace, and social) in order to assess temporal, situational and lifetime measures of exposure.
Materials and Methods
Study Population
The California Teachers Study (CTS) cohort was established from respondents to a 1995 mailing to all 329,000 active and retired female enrollees in the State Teachers Retirement System (STRS). STRS members include California public school employees who teach at the kindergarten through community college levels, are involved in the selection and preparation of instructional materials for these levels, or supervise persons engaged in these activities. Enrollment in the CTS with completed baseline questionnaires was 133,479. A full description of the CTS cohort is available elsewhere (5). Indicator data on active and passive smoking as well as extensive information on breast cancer risk factors were collected on the baseline survey. Because of the high prevalence of lifetime nonsmokers in the cohort (66%), and because of the current interest in passive smoking, more detailed questions on source, setting, timing and dose of passive smoking exposures were included in the second (Wave II) survey mailed in Fall, 1997. A total of 99,213 CTS members reported at least some information on passive smoking on the Wave II survey. The present analysis excluded women who ever smoked tobacco products (n= 33,223), were not residents of California at the time of completing their Wave II questionnaire (n=4,398), or who were diagnosed with breast cancer prior to completing the Wave II questionnaire (n= 4,069). The resulting study sample consisted of 57,523 women, of whom 1,754 were diagnosed with invasive breast cancer after completing their Wave II questionnaire and before December 31, 2007.
Use of human subjects data in this study was reviewed by the Human Subjects Research Committees of the Northern California Cancer Center, the City of Hope, the University of Southern California, the University of California at Irvine, and the California Health and Human Services Agency, and found to be in compliance with their ethical standards as well as with the U.S. Code of Federal Regulations, Title 45, Part 46 on the Protection of Human Subjects.
Outcome Assessment
The CTS cohort is followed annually for cancer diagnosis, death and change of address. Cancer outcomes are identified through annual linkage with the California Cancer Registry (CCR), a legally mandated statewide population-based cancer reporting system. Modeled after the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program, the CCR is comprised of three SEER Program registries and maintains the highest standards for data quality and completeness. CCR ascertainment of newly diagnosed cancers is estimated to be 99% complete and includes case-sharing from neighboring states (6). Cases of invasive breast cancer for this analysis were identified through annual linkages between the CTS cohort and the CCR database. Tumor hormone responsiveness was obtained from the CCR. Mortality files, as well as reports from relatives, are used to ascertain date and cause of death. Changes of address are obtained by annual mailings, responses from participants, and from record linkages using various online data resources, including the U.S. Postal Service National Change of Address database.
Calculation of Follow Up
Person-days at risk was calculated as the time between the date each woman completed her Wave II questionnaire and the earliest of four dates: her breast cancer diagnosis date, the date of her death, the date she moved out of California for a period longer than four consecutive months, or December 31, 2007, the end of follow-up. Women who were diagnosed with in situ breast cancer during follow-up were censored as of the date of diagnosis.
Assessing Passive Smoking Exposures in Lifetime Nonsmokers
Lifetime nonsmokers were identified based on a question from the baseline questionnaire as those women who responded negatively to the question of whether they had ever smoked 100 or more cigarettes during their lifetime. Mailed self-administered questionnaires in 1997 were used to collect information on lifetime passive smoking exposures for three settings (household, workplace and social) and six age periods (<20, 20-29, 30-39, 40-49, 50-59, and ≥60). For each combination of setting and age period, respondents were asked whether they were exposed to the tobacco smoke of others. Within each combination of setting and age period, passive smoke exposure among respondents who answered yes was further assessed along two separate dimensions: duration and intensity. Duration was estimated by asking for details regarding the number of years exposed (< 1 year, 1-3 years, 4-6 years, and 7+ years) within that age period and setting. Smoke intensity was estimated by a qualitative description of the smoke intensity in that setting during that age period: a little smoky, fairly smoky, or very smoky.
To facilitate subsequent analyses, the duration and intensity responses within each combination of setting and age period were transformed to numeric values. Because “years of exposure” were reported in ranges of years rather than a specific number of years, the midpoint of any year range was used to represent that range. For example, 5 years was used to represent the range 4-6 years. The qualitative smoke intensity responses were assigned a value of 1, 2, or 3 (1=a little smoky, 2=fairly smoky, 3=very smoky).
Because of the accumulating evidence that early life exposures may play a role in breast cancer etiology, we further characterized these exposures by the age period in which they were experienced. Within each setting, the numeric smoking data were aggregated into two broad mutually exclusive age periods: before age 20 years and age 20 years or greater (roughly approximating childhood/teenage vs. adult exposures). The duration for each broad age period was calculated by summing the numerical years of exposure of each contained smaller age period. Likewise, the intensity for each broad age period was calculated by averaging the numerical intensity score of each contained smaller age period.
Finally, the duration and intensity for the age periods less than 20 and 20 or greater were summed across the settings to get overall measures of duration (setting-specific years) and intensity for those two broad age periods.
Similar to Cummings (7), we combined the two individual metrics that best predicted breast cancer risk (years of exposure and intensity) into a common summary metric (intensity-years) that incorporated both intensity (smokiness) and duration (years) of exposure. For each respondent, a measure of “intensity-years” was created for each combination of setting and age period by multiplying the number of years and smoke intensity values for that setting and age period. These within-setting intensity-years measures for a respondent were then summed into the same two broad age groups as above: before age 20 and age 20 or greater, and then further summed across the settings to get overall measures of duration and intensity for those two age periods.
Overall “lifetime” exposure estimates for duration, intensity, and intensity-years for each respondent were obtained by summing the estimates for before age 20 and age 20 or greater for that respondent.
Cutpoints for exposure categories (in tertiles) were based on the distribution of each cumulative exposure measure among all those with non-zero values of passive smoking exposure.
The questionnaire did not directly ask about second hand smoke in relation to timing of first pregnancy. Women with exposures that clearly occurred prior to or after the pregnancy were categorized into ‘pre-first pregnancy’ and ‘post-first pregnancy’ exposure groups. Women with both pre- and post-first pregnancy exposures were categorized with the ‘pre-first pregnancy’ group. Because exposures were reported by decades and not individual years, pinpointing whether second hand smoke exposures clearly occurred before or after pregnancy was not possible for all respondents. In such cases, women were classified into an unknown timing of exposure category. An additional exposure category included women with no reported passive smoke exposure before or after a first full term pregnancy.
Personal Risk Factors
From the baseline and Wave II questionnaires, we collected information on the following personal breast cancer risk factors: age (calculated from date of birth and date of Wave II questionnaire completion); race/birthplace (white, non-white US or Canadian born, non-white non-US or Canadian born, and other/unknown); family (first-degree relative) history of breast cancer (yes, no, and adopted/not provided); women's age at menarche (less than 12 years old, 12-13 years old, 14 years old or older, and not provided); pregnancy history (nulliparous or parous, with four categories for age at first full-term pregnancy: less than 25 years old, 25-29 years old, 30 years old or older, and unknown age); breast feeding (nulliparous or parous, with six categories for total lifetime breast feeding months: never breast fed, less than 6 months, 6-11 months, 12 or more months, pregnant with no live birth, or unknown breast feeding history); physical activity, defined as the average number hours per week of strenuous activity over a woman's lifetime (less than ½ hour per week, ½ hour - 2 hours per week, greater than 2 hours - 3½ hours per week, greater than 3½ hours - 5 hours per week, greater than 5 hours per week, and not provided); current alcohol consumption, measured in grams per day (nondrinkers, consumers of less than 20 grams per day, 20 grams or more per day, and unknown/missing); body mass index (BMI) tertiles (16-25.7 kg/m2, 25.8-32.2 kg/m2, 32.3-54.8 kg/m2, and height or weight not provided or out or range coded to missing); menopausal status and hormone therapy (HT) combined (premenopausal, peri/postmenopausal and no HT, peri/postmenopausal and HT only in the past, peri/postmenopausal and current estrogen user, peri/postmenopausal and current estrogen and progestin user, all others). Menopausal status (pre-, peri-, postmenopausal, unknown status) was derived at the time of the baseline questionnaire from responses to questions regarding information about menstrual periods, duration and timing of both estrogen and progestin therapy, age of respondent, and ages at reported surgeries, if relevant.
Statistical Analyses
All analyses were performed using SAS 9.1 (8) or R 2.9.1 (9). Cox proportional hazards models were used to estimate hazard rate ratios (HRs) and 95% confidence intervals (CIs) associated with measures of passive smoking exposure, using age at start and end of follow-up (in days) to define time on study. No apparent violation of the underlying assumption of proportional hazards was detected. All models were stratified by age and either adjusted for race only or for race and personal risk factors of interest: family history of breast cancer, age at menarche, pregnancy history, lifetime duration of breast feeding, physical activity, alcohol consumption, BMI, and menopausal status combined with HT use categorization. All statistical tests were two-sided, and p values less than 0.05 were considered statistically significant. Where appropriate, linear tests for trend across categories of exposure were performed. Individual tests were not adjusted for multiple testing.
In all categorical analyses, the referent group consisted of those women never exposed to passive smoking. In analyses involving age-specific categorical exposures, i.e., age less than 20 or age 20 or more, main effects models were fit using a separate two-level factor (not exposed in that age range vs. exposed in that age range) for each of the two age ranges. Likewise for analyses involving setting-specific categorical exposures, i.e., home, work, or social settings, a main effects model with three two-level factors were used: not exposed vs. exposed for the three settings. For analyses involving tertiles of “years of exposure”, “intensity of exposure”, or “intensity-years of exposure”, the tertiles for each exposure were coded as four levels of a categorical variable: “None”, “Low”, “Medium”, and “High.” Main effects models for age-specific tertile analysis consisted of two four-level categorical variables, one variable for each specific age group.
The dose response analysis and the analysis assessing sensitivity to threshold were done using the survival package in R. For the dose response analysis, only subjects who reported an overall value exceeding 4.0 intensity-years, representing at least 4 years of mild intensity exposure were used (or fewer years with higher intensity). Adult intensity-years were represented in the model by a linear term, but on a log scale, to symmetrize the distribution of exposures. The model was stratified by age and adjusted for race. For the sensitivity analysis, the same model was repeatedly fit, while varying the exposure threshold that must be exceeded from zero to fifty intensity-years. Above 50 intensity-years, the model fitting procedure started to fail. For purposes of displaying the dose response curve only, the mgcv package in R was used to fit a binomial generalized additive model with logit link and a smoothing spline term for adult intensity-years. The model was adjusted for race and age, also represented as a smoothing spline. The smoothing spline was then graphed, along with its point wise confidence interval. The entire spline curve was shifted graphically so that the logit corresponding to the minimum exposure was zero.
Results
Similar to the entire CTS cohort, subjects in this analysis were predominantly non-Hispanic white (86%) and almost half were post-menopausal at baseline. Compared to those who did not have a breast cancer diagnosis, cases tended to be older and more frequently report a family history of breast cancer. The vast majority (76%) of tumors diagnosed among women in this analysis were hormone responsive.
The distributions of women according to their passive smoking exposure by timing and setting are presented in Table 1. Overall, 86% of study subjects reported some passive smoking exposure during their lifetime. Most reported household exposure (71%), half reported workplace exposure and more than one-third reported exposure in a social setting (37%). Approximately two thirds of respondents reported exposure before the age 20 and nearly three-quarters reported exposure at age 20 or older. Among those with exposures before age 20, the household was the predominant setting (58%), followed by considerably less exposure in social (20%) and workplace (17%) settings. In contrast, adult exposures (age 20 or older) were most commonly reported for the workplace (47%), followed closely by household (43%) and social settings (34%).
Table 1.
Passive smoke exposure by timing and setting among study participants (n=57,523).
| Exposure | Number Exposed Among | ||
|---|---|---|---|
| Non-cases N |
Breast Cancer Cases N |
Entire Study Population N (%) |
|
| Any lifetime exposure* | 47,901 | 1,567 | 49,468 (86) |
| Any household lifetime exposure | 39,461 | 1,312 | 40,773 (71) |
| Any workplace lifetime exposure | 27,623 | 958 | 28,581 (50) |
| Any social lifetime exposure | 20,692 | 698 | 21,390 (37) |
| Any age < 20 exposure* | 36,286 | 1,150 | 37,436 (65) |
| Any age <20 household exposure | 32,412 | 1,040 | 33,452 (58) |
| Any age <20 workplace exposure | 9,503 | 295 | 9,798 (17) |
| Any age <20 social exposure | 11,010 | 356 | 11,366 (20) |
| Any age ≥20 exposure* | 39,848 | 1,363 | 41,211 (72) |
| Any age ≥20 household exposure | 23,694 | 864 | 24,558 (43) |
| Any age ≥20 workplace exposure | 25,944 | 914 | 26,858 (47) |
| Any age ≥20 social exposure | 18,870 | 646 | 19,516 (34) |
Exposure categories are not mutually exclusive and therefore individual setting totals will not sum to “any exposure.”
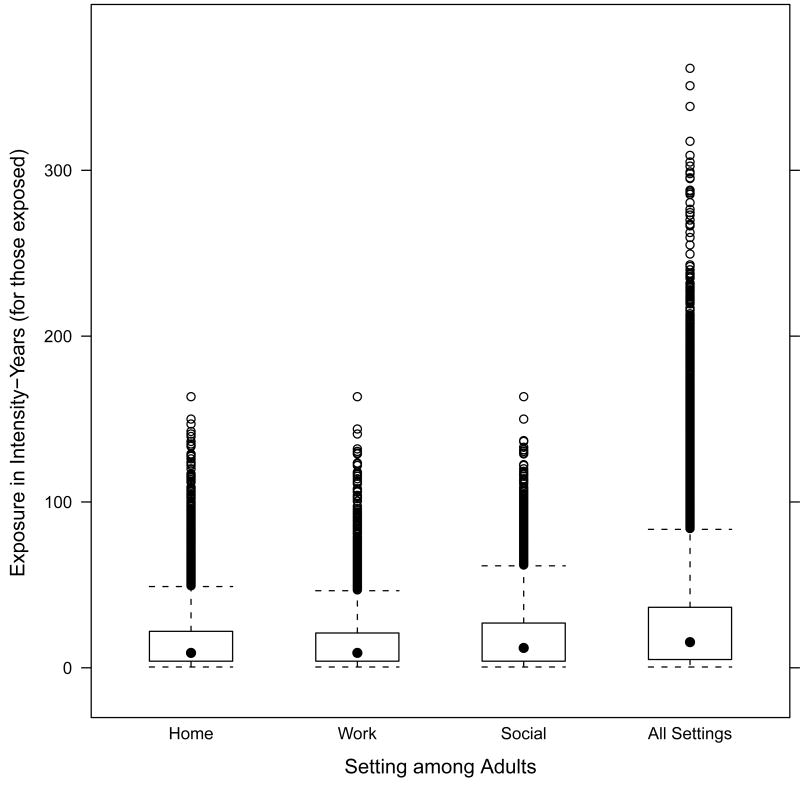
The distributions of adult exposures in the three setting are shown in Figure 1. The distributions of exposures in the workplace and home, among those exposed, were virtually identical, and both were lower than the distribution of exposures in social settings. The final box plot in Figure 1 shows the distribution of the sum of the intensity-years measures across the three settings, where the large upper tail of the “All Settings” box plot reflects exposure levels that would not be captured by examining household exposures only. Moreover, the workplace exposures appear to be at least as intense as home exposures.
Figure 1.
Distributions of intensity-years among adults for three settings. The box shows the 25th and 75th percentiles, the dot shows the median, the whiskers above and below the box show the range of the bulk of the distribution, and the dots show extreme values. The range of exposures is cut off at 400, removing one All Settings observation at 490.5.
Exposures by setting and age group were not mutually exclusive, although correlations were modest. The Spearman correlation coefficient(r) for the intensity-years measure, for instance, was 0.25 for overall childhood and adulthood exposures (among those exposed in both ages). For adult exposures, the Spearman r was 0.23 for home and work exposures, and 0.39 for work and social settings (among those exposed in all three settings).
The point estimates for risk of breast cancer associated with any exposure (ever/never) to passive smoking reported by study participants did not substantially differ from the null in any of the age group or setting categories, and risk estimates from age stratified and race/birthplace adjusted models were similar to those from the models fully adjusted for personal risk factors, albeit with wider confidence intervals (Table 2). Although not statistically significant, the highest point estimates were observed for lifetime exposure opportunity, rather than for any particular setting or age group.
Table 2.
Invasive breast cancer Hazard Ratios (HRs) and 95% Confidence Intervals (95% CI) associated with never/ever passive smoking exposures, by setting and by age of exposure, among 1,754 cases diagnosed 1997-2007 in 57,523 study participants.
| Passive Smoke Exposure | N | #cases | HR | 95% CI | HR | 95% CI |
|---|---|---|---|---|---|---|
| Adjusted for age and race | Adjusted for age, race and personal risk factors* | |||||
| Lifetime exposure: | ||||||
| Never | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Ever | 49,468 | 1,567 | 1.13 | 0.96, 1.33 | 1.10 | 0.94, 1.30 |
| Age-Specific Exposures: | ||||||
| Never | 7,070 | 163 | 1.00 | |||
| Any Age < 20 Exposure | 37,436 | 1,150 | 1.06 | 0.94, 1.19 | 1.06 | 0.94, 1.19 |
| Any Age ≥ 20 Exposure | 41,211 | 1,363 | 1.06 | 0.93, 1.21 | 1.04 | 0.91, 1.19 |
| Setting-Specific Exposures: | ||||||
| Never | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Any Home Exposure | 40,773 | 1,312 | 1.05 | 0.93, 1.17 | 1.04 | 0.92, 1.16 |
| Any Work Exposure | 28,581 | 958 | 1.03 | 0.94, 1.14 | 1.02 | 0.93, 1.13 |
| Any Social Exposure | 21,390 | 698 | 1.01 | 0.91, 1.11 | 1.00 | 0.90, 1.10 |
Personal risk factors include family history of breast cancer, age at menarche, pregnancy history, lifetime duration of breastfeeding, physical activity, alcohol consumption, body mass index, and categories of menopausal status with use of hormone therapy.
Analyses evaluating the cumulative measures for each component of exposure (years and intensity) contributing to the summary measure of intensity-years and the summary measure itself generally did not show consistent and significant associations for particular settings or for overall lifetime exposures or for those under age 20 years (Table 3). Point estimates were significantly elevated for the highest exposure categories among women exposed at ages 20 years or older for years and intensity-years in the age/race adjusted models, but remained statistically significant in the fully adjusted models only for the combined summary measure, intensity-years (HR=1.18, 95% CI=1.00,1.40). A test for trend, however, was not statistically significant (p=0.30).
Table 3.
Invasive breast cancer Hazard Ratios (HRs) and 95% Confidence Intervals (95% CI) associated with years, intensity, and intensity-years of passive smoke exposure for 1,754 cases diagnosed 1997-2007 among 57,523 study participants.
| Exposure | N | #cases | HR | 95% CI | HR | 95% CI |
|---|---|---|---|---|---|---|
| Adjusted for age and race | Adjusted for age, race and personal risk factors* | |||||
| Years:** | ||||||
| Lifetime Exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Low (≤15.0) | 16,551 | 436 | 1.12 | 0.94, 1.34 | 1.10 | 0.92, 1.32 |
| Medium (15.1 – 30.0) | 16,314 | 495 | 1.13 | 0.94, 1.35 | 1.10 | 0.92, 1.32 |
| High (>30.0) | 16,299 | 629 | 1.14 | 0.96, 1.36 | 1.12 | 0.93, 1.33 |
| Age-Specific Exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Age <20 exposure: | ||||||
| Low (≤15.0) | 14,719 | 441 | 1.13 | 0.99, 1.29 | 1.13 | 0.99, 1.29 |
| Medium (15.1 – 30.0) | 19,167 | 591 | 0.99 | 0.87, 1.13 | 0.99 | 0.87, 1.13 |
| High (>30.0) | 3,017 | 100 | 0.99 | 0.79, 1.24 | 0.98 | 0.78, 1.23 |
| Age ≥20 exposure: | ||||||
| Low (≤15.0) | 22,938 | 633 | 1.06 | 0.92, 1.22 | 1.04 | 0.90, 1.20 |
| Medium (15.1 – 30.0) | 8,888 | 308 | 1.01 | 0.86, 1.20 | 1.00 | 0.84, 1.18 |
| High (>30.0) | 8,934 | 409 | 1.19 | 1.01, 1.41 | 1.16 | 0.98, 1.38 |
| Intensity: | ||||||
| Lifetime Exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Low (≤2.0) | 25,544 | 778 | 1.11 | 0.94, 1.31 | 1.09 | 0.92, 1.29 |
| Medium (2.1 – 3.0) | 9,330 | 292 | 1.11 | 0.92, 1.35 | 1.08 | 0.89, 1.31 |
| High (>3.0) | 13,193 | 446 | 1.18 | 0.98, 1.41 | 1.14 | 0.95, 1.37 |
| Age-Specific Exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Age <20 exposure: | ||||||
| Low (≤2.0) | 26,243 | 820 | 1.07 | 0.95, 1.21 | 1.07 | 0.95, 1.21 |
| Medium (2.1 – 3.0) | 4,814 | 131 | 0.94 | 0.77, 1.15 | 0.95 | 0.77, 1.16 |
| High (>3.0) | 5,015 | 159 | 1.03 | 0.84, 1.26 | 1.04 | 0.85, 1.28 |
| Age ≥20 exposure: | ||||||
| Low (≤ 2.0) | 22,764 | 714 | 1.04 | 0.91, 1.20 | 1.03 | 0.89, 1.18 |
| Medium (2.1 – 3.0) | 7,469 | 244 | 1.08 | 0.90, 1.29 | 1.04 | 0.87, 1.24 |
| High (>3.0) | 9,341 | 346 | 1.17 | 0.98, 1.39 | 1.13 | 0.95, 1.34 |
| Intensity-years: | ||||||
| Lifetime exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Low (≤17.5) | 17,618 | 483 | 1.12 | 0.94, 1.34 | 1.10 | 0.92, 1.31 |
| Medium (17.6 – 42.0) | 14,356 | 447 | 1.13 | 0.94, 1.35 | 1.10 | 0.92, 1.32 |
| High (>42.0) | 15,886 | 580 | 1.14 | 0.96, 1.36 | 1.11 | 0.93, 1.32 |
| Age-Specific Exposures: | ||||||
| None | 7,070 | 163 | 1.00 | - | 1.00 | - |
| Age <20 exposure: | ||||||
| Low (≤17.5) | 21,375 | 673 | 1.09 | 0.96, 1.23 | 1.09 | 0.96, 1.23 |
| Medium (17.6 – 42.0) | 10,156 | 295 | 0.97 | 0.83, 1.13 | 0.97 | 0.83, 1.13 |
| High (>42.0) | 4,195 | 127 | 0.99 | 0.80, 1.22 | 0.99 | 0.80, 1.22 |
| Age ≥20 exposure: | ||||||
| Low (≤ 17.5) | 21,514 | 604 | 1.06 | 0.92, 1.22 | 1.04 | 0.90, 1.20 |
| Medium (17.6 – 42.0) | 9,213 | 303 | 1.01 | 0.85, 1.19 | 0.99 | 0.83, 1.17 |
| High (>42.0) | 8,599 | 389 | 1.22 | 1.03, 1.44 | 1.18 | 1.00, 1.40 |
Personal risk factors include family history of breast cancer, age at menarche, pregnancy history, lifetime duration of breastfeeding, physical activity, alcohol consumption, body mass index, and categories of menopausal status with use of hormone therapy.
“Years” represents the sum of years of exposure across the three categories of home, work and social.
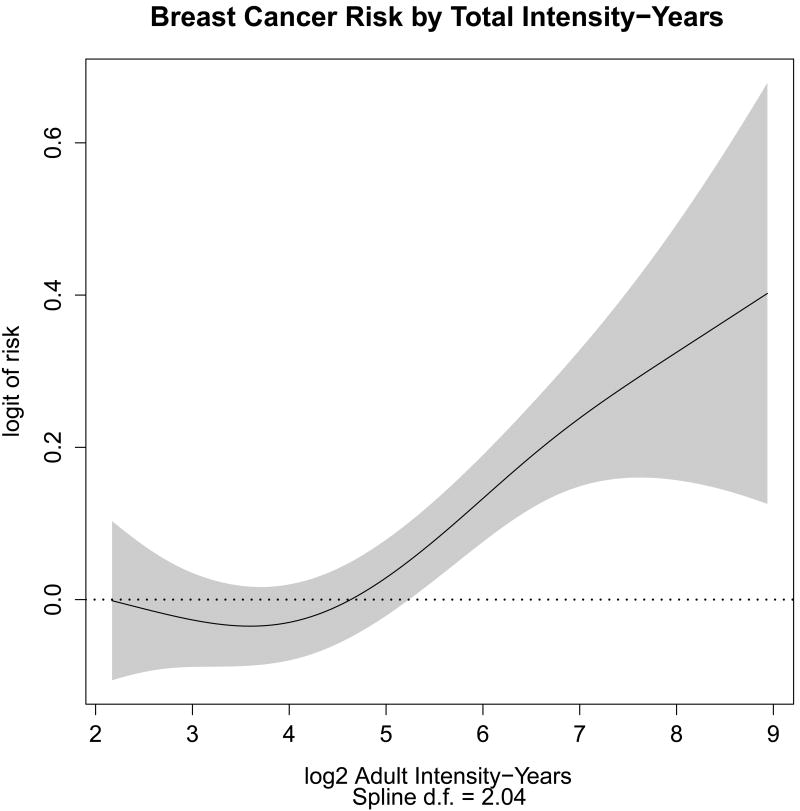
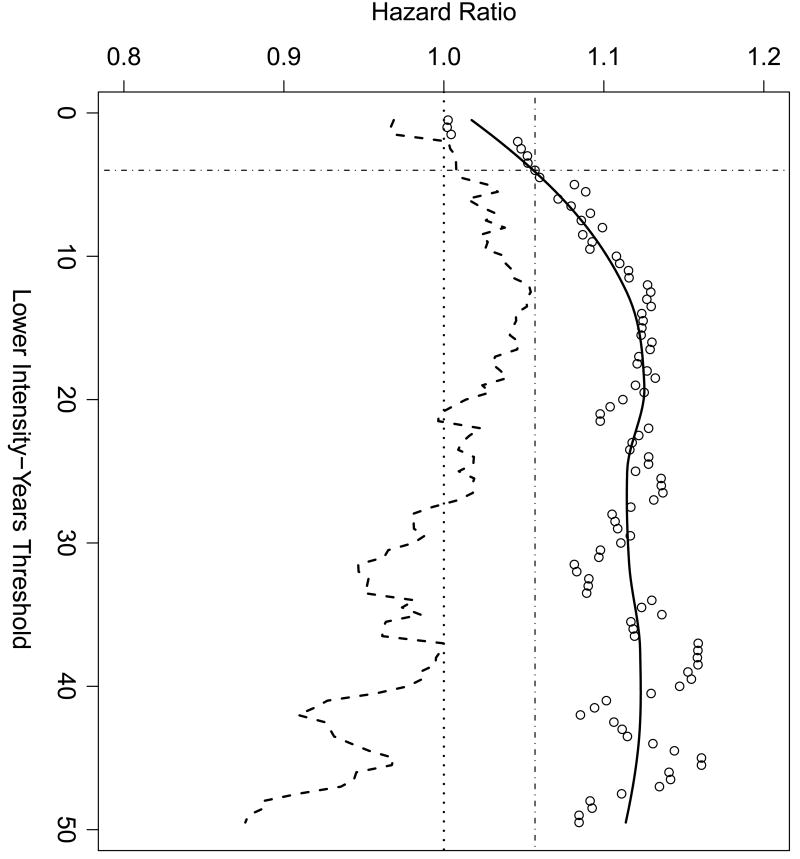
When attention was confined to those subjects with a modest to large exposure to environmental tobacco smoke (total intensity-years > 4 for all exposures), a statistically significant nonlinear dose response curve emerged for exposure modeled on a log scale (Figure 2). After stratifying by age and adjusting for race, log2 intensity-years had a marginally statistically significant linear term (HR=1.06, 95% CI=1.01, 1.11, p=0.02), corresponding to a 6% increase in hazard for every doubling of intensity-years. Figure 3 shows how the estimated slope of a linear dose response curve in log2 years changes as we vary the threshold used to confine our attention. As the lower-exposed subjects are eliminated from consideration by increasing the threshold for “modest to large” exposures, the linear part of the dose-response increases as well, reaching an asymptote of around 1.13 at a threshold of approximately 20 intensity-years. Hence, for exposures exceeding 20 intensity-years, a doubling of exposure results in an approximately 13% increase in hazard. At that point, fitting becomes more and more unstable as the number of subjects diminishes. To relate this result back to Figure 2, note that the thin vertical line in Figure 3, corresponding to a threshold of 4, intercepts the smoothed hazard curve at a value of approximately 1.06, corresponding to the 1.06 calculated above. As expected with a dose-response curve that is concave at lower doses, restricting one's attention to dose-response curves that are linear in log2 intensity-years biases the effect estimate for doubling large exposures downward.
Figure 2.
Smoothing spline representation of the dose response curve for total intensity-years in adults from all sources. The curve represents the logit of relative risk as a function of dose, was calculated using a binomial Generalized Additive Model, and arbitrarily translated so that the logit corresponding to the lowest exposure is zero. The subjects are restricted to those with a Intensity-years score from all sources exceeding 4. The shaded area represents the point wise 95% confidence interval, and the dotted line shows response of zero.
Figure 3.
The effect of changing the lower threshold for inclusion in the fit on the coefficient of the slope term in a proportional hazards model relating breast cancer to log2 intensity-years. Each dot represents an estimated hazard ratio for proportional hazards fit. The horizontal axis gives the lower inclusion threshold, the vertical axis gives the computed hazard ratio, and the dashed line shows the lower confidence interval for the fit. The solid line is a scatterplot smoother fit to the points. The thin vertical line marks the estimated slope when the threshold is 4, the value used in Figure 2. The thin horizontal line at 1.06 marks the hazard ratio corresponding a threshold of 4, as noted in the text.
Risks stratified by menopausal status are presented in Table 4. Women who were peri-menopausal at baseline were excluded from this analysis, as the number of events in this group was too small for reliable point estimates. There was no evidence for elevated breast cancer risks from passive smoking among pre-menopausal women at baseline. Separate analyses for women who were pre-menopausal at entry but were followed for a cancer event only up to the age of 50 (as a proxy for menopause), produced slightly elevated but non-statistically significant risk estimates for the highest level of cumulative lifetime exposure (HR=1.20; 95% CI 0.70 – 2.05), based on 33 cases in this category of exposure. For postmenopausal women, hazard ratios for lifetime low, medium and high cumulative exposure were 1.17 (95% CI 0.91, 1.49), 1.19 (95% CI 0.93, 1.53, and 1.26 (95% CI 0.99, 1.60), respectively.
Table 4.
Invasive breast cancer Hazard Ratios (HRs) and 95% Confidence Intervals (95% CI), by menopausal status at baseline, associated with any exposure and intensity-years of passive smoke exposure in the California Teachers Study.
| Menopausal Status at Baseline* | N | #cases | HR | 95% CI | HR | 95% CI |
|---|---|---|---|---|---|---|
| Adjusted for age and race | Adjusted for age, race and personal risk factors** | |||||
| Pre-Menopausal at Baseline | ||||||
| Any Exposure | ||||||
| Lifetime exposure | ||||||
| Never | 4,030 | 59 | 1.00 | - | 1.00 | - |
| Ever | 21,510 | 404 | 1.04 | 0.79, 1.38 | 1.04 | 0.79, 1.38 |
| Age-specific exposure | ||||||
| Never | 4,030 | 59 | 1.00 | - | 1.00 | - |
| Ever exposed <age 20 | 17,636 | 324 | 0.96 | 0.77, 1.18 | 0.95 | 0.77, 1.18 |
| Ever exposed ≥age 20 | 16,259 | 307 | 0.96 | 0.77, 1.19 | 0.95 | 0.77, 1.19 |
| Intensity-years | ||||||
| Lifetime exposure | ||||||
| None | 4,030 | 59 | 1.00 | - | 1.00 | - |
| Low (≤17.5) | 9,686 | 176 | 1.11 | 0.83, 1.50 | 1.11 | 0.82, 1.49 |
| Medium (17.6 – 42.0) | 6,587 | 130 | 1.06 | 0.77, 1.44 | 1.06 | 0.77, 1.44 |
| High (>42.0) | 4,808 | 90 | 0.92 | 0.66, 1.28 | 0.92 | 0.66, 1.29 |
| Age-specific exposure | ||||||
| None | 4,030 | 59 | 1.00 | - | 1.00 | - |
| Age <20 years exposure | ||||||
| Low (≤17.5) | 10,390 | 186 | 0.97 | 0.77, 1.22 | 0.96 | 0.76, 1.21 |
| Medium (17.6 – 42.0) | 4,788 | 95 | 1.02 | 0.77, 1.34 | 1.02 | 0.78, 1.36 |
| High (>42.0) | 2,019 | 31 | 0.77 | 0.51, 1.16 | 0.77 | 0.51, 1.16 |
| Age ≥20 years exposure | ||||||
| Low ((≤17.5) | 11,528 | 208 | 0.98 | 0.78, 1.23 | 0.97 | 0.77, 1.22 |
| Medium (17.6 – 42.0) | 2,975 | 62 | 0.96 | 0.69, 1.32 | 0.95 | 0.69, 1.31 |
| High (>42.0) | 1,257 | 30 | 0.98 | 0.64, 1.50 | 0.98 | 0.64, 1.51 |
| Pre-Menopausal at Baseline and Under age 50 at Follow-up*** | ||||||
| Any Exposure | ||||||
| Lifetime exposure | ||||||
| Never | 2,235 | 32 | 1.00 | - | 1.00 | - |
| Ever | 7,843 | 160 | 0.89 | 0.60, 1.33 | 0.93 | 0.61, 1.41 |
| Age-specific exposure | ||||||
| Never | 2,235 | 32 | 1.00 | - | 1.00 | - |
| Ever exposed <age 20 | 6,550 | 129 | 0.93 | 0.65, 1.31 | 1.02 | 0.71, 1.48 |
| Ever exposed ≥age 20 | 5,366 | 119 | 0.94 | 0.66, 1.32 | 0.90 | 0.63, 1.29 |
| Intensity-years | ||||||
| Lifetime exposure | ||||||
| None | 2,235 | 32 | 1.00 | - | 1.00 | - |
| Low (≤17.5) | 4,313 | 79 | 0.90 | 0.58, 1.40 | 0.91 | 0.58, 1.43 |
| Medium (17.6 – 42.0) | 2,226 | 44 | 0.75 | 0.46, 1.22 | 0.80 | 0.48, 1.34 |
| High (>42.0) | 1,190 | 33 | 1.13 | 0.68, 1.89 | 1.20 | 0.70, 2.05 |
| Age-specific exposure | ||||||
| None | 2,235 | 32 | 1.00 | - | 1.00 | - |
| Age <20 years exposure | ||||||
| Low (≤17.5) | 4,109 | 69 | 0.82 | 0.55, 1.21 | 0.87 | 0.57, 1.31 |
| Medium (17.6 – 42.0) | 1,656 | 40 | 1.07 | 0.67, 1.70 | 1.20 | 0.74, 1.95 |
| High (>42.0) | 649 | 14 | 1.10 | 0.57, 2.13 | 1.15 | 0.58, 2.28 |
| Age ≥20 years exposure | ||||||
| Low (≤17.5) | 4,501 | 90 | 0.87 | 0.61, 1.25 | 0.80 | 0.55, 1.17 |
| Medium (17.6 – 42.0) | 660 | 18 | 1.00 | 0.55, 1.81 | 1.12 | 0.60, 2.07 |
| High (>42.0) | 89 | 6 | 1.15 | 0.43, 3.05 | 0.98 | 0.34, 2.79 |
| Post-menopausal at Baseline | ||||||
| Any Exposure | ||||||
| Lifetime exposure | ||||||
| Never | 2,545 | 84 | 1.00 | - | 1.00 | - |
| Ever | 23,002 | 989 | 1.25 | 1.00, 1.57 | 1.22 | 0.97, 1.52 |
| Age-specific exposure | ||||||
| Never | 2,545 | 84 | 1.00 | - | 1.00 | - |
| Ever exposed <age 20 | 15,857 | 688 | 1.12 | 0.96, 1.30 | 1.12 | 0.97, 1.31 |
| Ever exposed ≥age 20 | 20,774 | 910 | 1.15 | 0.95, 1.39 | 1.11 | 0.92, 1.34 |
| Intensity-years | ||||||
| Lifetime exposure | ||||||
| None | 2,545 | 84 | 1.00 | - | 1.00 | - |
| Low (≤17.5) | 6,281 | 251 | 1.19 | 0.93, 1.52 | 1.17 | 0.91, 1.49 |
| Medium (17.6 – 42.0) | 6,270 | 264 | 1.23 | 0.96, 1.57 | 1.19 | 0.93, 1.53 |
| High (>42.0) | 9,393 | 430 | 1.31 | 1.03, 1.65 | 1.26 | 0.99, 1.60 |
| Age-specific exposure | ||||||
| None | 2,545 | 84 | 1.00 | - | 1.00 | - |
| Age <20 exposure | ||||||
| Low (≤17.5) | 8,900 | 411 | 1.16 | 0.99, 1.37 | 1.17 | 0.99, 1.37 |
| Medium (17.6 – 42.0) | 4,168 | 161 | 0.95 | 0.77, 1.16 | 0.96 | 0.78, 1.18 |
| High (>42.0) | 1,654 | 77 | 1.13 | 0.86, 1.48 | 1.14 | 0.87, 1.50 |
| Age ≥20 exposure | ||||||
| Low (≤17.5) | 7,768 | 325 | 1.15 | 0.93, 1.41 | 1.12 | 0.91, 1.37 |
| Medium (17.6 – 42.0) | 5,211 | 205 | 1.06 | 0.84, 1.32 | 1.02 | 0.81, 1.28 |
| High (>42.0) | 6,563 | 327 | 1.31 | 1.05, 1.62 | 1.25 | 1.01, 1.56 |
There were too few cases in the peri-menopausal group at baseline to include in these analyses.
Personal risk factors include family history of breast cancer, age at menarche, pregnancy history, lifetime duration of breastfeeding, body mass index, physical activity, alcohol consumption, and use of hormone therapy.
Premenopausal at baseline and follow up includes women who were premenopausal at baseline and either diagnosed or censored before the age of 50.
Analyses evaluating categories of exposure separately by setting (household, workplace and social) for those exposures occurring at ages 20 years or older yielded globally null results (data not shown). Those who reported workplace exposures, the predominant source of exposure during this age period, in the medium and highest categories had only slightly elevated risk estimates with confidence limits that included 1.0 (HR=1.05 in both categories) compared to those with no reported workplace exposure.
Passive smoking exposure in relation to a women's first pregnancy was categorized among parous subjects in the study (n=44,680). A slightly elevated risk of breast cancer was observed for women with known passive smoking exposure prior to a first pregnancy in age stratified and race/birthplace adjusted models (HR 1.21, 95% CI 1.00-1.47), as well as the fully adjusted models (HR 1.17. 95% CI 0.96-1.41).
Finally, we conducted a number of stratified analyses to evaluate whether risks differed by family history of breast cancer, or tumor hormone responsiveness. Results from these analyses did not vary by subgroup and were similar to those observed in the full study sample (data not shown).
Discussion
Our results suggest that cumulative exposures during adulthood to high levels of passive smoke may increase risk for breast cancer, particularly among postmenopausal women. Among women exposed to second hand smoke in adulthood (ages 20 years or older), modest but consistent elevations in risk were evident for women with the highest cumulative levels of adult exposure across settings. This observation was reinforced by a statistically significant dose response for analyses restricted to women with moderate to high levels of passive smoke exposure. The nonlinear nature of the dose-response shown in Figure 2, along with the sensitivity analysis in Figure 3, shows that much care must be exercised in determining and validating the functional form of any putative dose response estimate.
Consistent with our findings in an earlier study in the CTS (4), reported household passive smoke exposure, even at greater levels of detail, did not alone appear to be associated with an increased risk for subsequently developing breast cancer among women who were lifetime nonsmokers. Likewise, neither evidence for a risk association separately by other settings of exposure (workplace or social), nor for early life exposures (under age 20 years) was apparent. Our findings of a suggestively, albeit non-statistically significant, increased risk for the highest category of intensity-years among premenopausal women at baseline and under the age of 50 at diagnosis (HR=1.20; 95% CI 0.70-1.05), is consistent with an earlier analysis from the same cohort that reported a similar risk for women based on a more crude measure of exposure (i.e., never/ever exposed during childhood and adulthood) among women under the age of 50 at diagnosis (HR=1.27; 95% CI 0.84 – 1.92) (10)
The body of literature on this topic is still relatively small and findings to date have not been consistent. To date there have been ten prospective cohort studies (4, 11-19) and 17 case-control studies (20-37) conducted to examine the relationship between passive smoking and breast cancer. Results have been mixed, with four of the ten cohort studies yielding positive results and 11 of the 17 case-control studies reporting positive findings. The most recent and largest of these is the prospective Million Women Study from the U.K. (19). The authors reported an overall null association (RR=0.98, 95% CI=0.93-1.05) for passive smoking and breast cancer, and the point estimate for risk in pre-menopausal women actually suggested an inverse association 0.54 (95% CI 0.33-0.99). The Million Women Study, however, was limited to women aged 53-67, collected information only for currently living with a smoking spouse, and found a mere 11% of respondents to be exposed by this criterion.
Inconsistencies in exposure assessment methods are likely to have greatly contributed to the observed inconsistencies in findings across studies. Early passive smoking studies relied on a husband's smoking history as the index of exposure, thus limiting analysis primarily to adult household exposures (13, 15, 27). However, as more women entered the workforce in the latter part of the last century, this measure missed the substantial contribution of workplace exposures (38). In the present analysis, workplace constituted the most prevalent setting for passive smoke exposure in adulthood for this occupational cohort. This is consistent with the fact that, prior to the enactment of restrictive legislation, California workplaces were a likely source of some fairly significant passive smoking exposures (7), and teachers have repeatedly told us that prior to that time teachers' lounge areas were heavily polluted with tobacco smoke.
More recent studies have focused on ascertaining detailed measures of passive smoking exposures outside the home (12, 24, 26) and have considered childhood exposures (4, 12, 14, 18, 20, 22-24, 33). Only two cohort studies have been published to date that have been able to characterize passive smoke exposure in settings other than the home and for a variety of time periods (12, 18). Both were relatively small studies of Japanese women that included measures of household and public exposures. One reported an odds ratio of 2.6 (95% CI 1.3-5.2) for passive smoking exposure among pre-menopausal women, but no association for post-menopausal women (12); the other reported no significant association between passive smoking and breast cancer (18).
Recent meta-analyses have suggested that the effect of passive smoking on breast cancer risks may be primarily limited to pre-menopausal women (39-41). Our study, however, is not alone nor the only cohort study in finding passive smoking risk associations among post-menopausal or primarily post-menopausal women (13, 15, 21, 22, 24, 26, 30, 42, 43). It is worth noting that these include three case-control studies with more complete exposure methods (21, 24, 43). In a large case-control study conducted in Shanghai risk was elevated only for workplace exposures among post-menopausal women, with a significant trend and an odds ratio of 1.6 (95% CI 1.0-2.5) for the highest exposure level (26). A Canadian study (24) incorporating both household and workplace exposures found increasing risk for postmenopausal women by level of exposure, although the point estimates were not as elevated as those among pre-menopausal women.
Our inability to detect increased risk among pre-menopausal women could be due to a number of factors. It is possible that there is an effect but we were unable to detect it because of small numbers and difficulties in accurately classifying changes in menopausal status. Because of the prospective nature of our study we could not accurately pinpoint menopausal status during follow-up among women who were premenopausal at baseline and outcomes were more heavily weighted for postmenopausal events in this aging cohort. Our analyses censoring observations at age 50 (to approximate menopausal status during follow-up) suggested a potentially elevated risk for the highest category of lifetime cumulative exposure among women who remained pre-menopausal during follow-up, but the number of cases in this category of women was very small. Perhaps more germane, younger study participants were less likely than older participants to have been subjected to extended workplace exposures as California restrictions on public and workplace smoking, some of the earliest in the nation, came into effect in the decade prior to study initiation. Post-menopausal women in this study, for example reported nearly three times more workplace exposures than did pre-menopausal women (an average of 8.4 years versus 2.9 years, respectively). This is larger than the differences for household exposures which were only about 50% higher among post- than among pre-menopausal women (an average of 16.5 years compared to 10.1 years). This, together with the fact that California's rates of active smoking have traditionally been lower than national averages during an era of declining public acceptance of smoking, may have resulted in exposure levels much lower than those experienced in other study populations. Indeed, if passive smoking acts as a promoter late in the process of carcinogenesis, then the rapidly changing exposure climate as a result of smoke free laws in the early 1990's would have been likely to reduce the observed relative risks for cases diagnosed during our study period.
Notably, one of the strongest arguments for the association between active smoking and breast cancer has come from the emerging literature accounting for genetic polymorphisms influencing tobacco metabolism. In particular, the recent extensive pooled analysis and meta-analysis conducted by Ambrosone and colleagues (44) of studies which have examined tobacco related risks in the context of the N-acetyltransferase 2 (NAT2) genotypes was cited by the Canadian reviewers as adding great credence to a causal role for active smoking. Few studies have evaluated the effect of NAT2 variants in studies of passive smoking and breast cancer. Two case control studies have suggested no modification of observed main effects for rapid vs. slow acetylators (29, 45), but a German case-control study suggested that, contrary to the observed relationship with active smoking in which slow acetylation is associated with greater risk, passive smoking was associated with higher risk in rapid acetylators (46). Although there have been some interesting, if null, studies of passive smoking and other polymorphisms (35, 47, 48) this remains a relatively uncharted area of inquiry.
The CTS study is one of the largest studies of passive smoking and breast cancer conducted to date. Because of the detailed information collected in the second CTS questionnaire, we have been able to explore risk associations by setting, for adulthood vs. childhood and adolescent years, for distinctive time periods, and for targeted subgroups of interest in order to inform the debate about whether passive smoke exposures may be associated with a higher risk of breast cancer. Likewise, because the CTS has collected detailed information on other risk factors for breast cancer, we were able to evaluate passive smoking associations independent of known risk factors. Furthermore, the prospective nature of the CTS precludes problems of differential recall bias. Because of the many comparisons in this study, these results could be due to chance. Nonetheless, the observed pattern of point estimates suggest a modest elevation in risk associated with higher levels of cumulative exposure during adulthood, particularly in post-menopausal women. The lack of an effect for specific settings or age groups may not be surprising if there is a true association with higher cumulative levels of exposure. This underscores the importance of collecting highly-detailed exposure information across all settings and for the course of a lifetime.
Although genotyping information on polymorphisms most relevant to tobacco metabolism is not yet available for the CTS, future work with these data may offer a clearer picture of the modest risk relationships from this observational analysis. Passive smoking has been implicated as a cause of lung cancer in lifetime nonsmokers. The question of whether these avoidable sources of exposure may contribute to the development of breast cancer, the leading cancer in women, remains an important issue.
Footnotes
Financial Support: This work was supported by the California's Tobacco Related Research Program (Grant # TRDRP 13RT-0018). National Cancer Institute (Grant #R01 CA77398), and contract 97-10500 from the California Breast Cancer Research Fund. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute.
The ideas and opinions expressed herein are those of the authors and endorsement by the California Department of Public Health, the National Cancer Institute, the Centers for Disease Control and Prevention, or their contractors and subcontractors is not intended nor should be inferred. The authors would like to thank the other CTS Steering Committee members who were not named as authors on this paper for their contributions to and support of the CTS: Hoda Anton-Culver, Ellen Chang, Christina A. Clarke, Rosemary Cress, Dennis Deapen, Pamela Horn-Ross, Susan Neuhausen, Rich Pinder, Daniel O. Stram, Giske Ursin, Dee W. West, and Argyrios Ziogas, and the women participating as members of the CTS cohort.
References
- 1.World Health Organization, International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Volume 83 -- Tobacco Smoke and Involuntary Smoking. Lyon, France: IARC Press; 2004. [PMC free article] [PubMed] [Google Scholar]
- 2.United States Public Health Service, Office of the Surgeon General. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Office of the Surgeon General; 2006. [Google Scholar]
- 3.Collishaw NE, Boyd NF, Cantor KP, et al. Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk. Toronto, Canada: Ontario Research Unit; 2009. (OTRU Special Report Series). [Google Scholar]
- 4.Reynolds P, Hurley S, Goldberg DE, et al. Active smoking, household passive smoking, and breast cancer: evidence from the California Teachers Study. J Natl Cancer Inst. 2004;96:29–37. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–35. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 6.Kwong SL, Perkins CI, Morris CR, Cohen R, Allen M, Wright WE. Cancer in California, 1988-1999. Sacramento, CA: California Department of Health Services, Cancer Surveillance Section; 2001. [Google Scholar]
- 7.Cummings KM, Markello SJ, Mahoney MC, Marshall JR. Measurement of lifetime exposure to passive smoke. Am J Epidemiol. 1989;130:122–32. doi: 10.1093/oxfordjournals.aje.a115303. [DOI] [PubMed] [Google Scholar]
- 8.SAS 9 [Computer Program] Version 9. Cary, NC: SAS Institute Inc.; 2007. [Google Scholar]
- 9.Ihaka R, Gentleman R. R: A language for data analysis and graphics. Journal of Computational and Graphical Statistics. 1996;5:299314. [Google Scholar]
- 10.Reynolds P, Hurley S, Goldberg D. CTS Steering Committee. Response to “Re: Active Smoking, Household Passive Smoking and Breast Cancer: Evidence from the California Teachers Study” [letter] JNCI. 2004 July 7;96(13):1042. doi: 10.1093/jnci/djh002. [DOI] [PubMed] [Google Scholar]
- 11.Gram IT, Braaten T, Terry PD, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev. 2005;14:61–6. [PubMed] [Google Scholar]
- 12.Hanaoka T, Yamamoto S, Sobue T, Sasaki S, Tsugane S. Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int J Cancer. 2005;114:317–22. doi: 10.1002/ijc.20709. [DOI] [PubMed] [Google Scholar]
- 13.Jee SH, Ohrr H, Kim IS. Effects of husbands' smoking on the incidence of lung cancer in Korean women. Int J Epidemiol. 1999;28:824–8. doi: 10.1093/ije/28.5.824. [DOI] [PubMed] [Google Scholar]
- 14.Egan KM, Stampfer MJ, Hunter D, et al. Active and passive smoking in breast cancer: prospective results from the Nurses' Health Study. Epidemiology. 2002;13:138–45. doi: 10.1097/00001648-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Hirayama T. Cancer mortality in nonsmoking women with smoking husbands based on a large-scale cohort study in Japan. Prev Med. 1984;13:680–90. doi: 10.1016/s0091-7435(84)80017-1. [DOI] [PubMed] [Google Scholar]
- 16.Nishino Y, Tsubono Y, Tsuji I, et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. doi: 10.1023/a:1012273806199. [DOI] [PubMed] [Google Scholar]
- 17.Wartenberg D, Calle EE, Thun MJ, Heath CW, Jr, Lally C, Woodruff T. Passive smoking exposure and female breast cancer mortality. J Natl Cancer Inst. 2000;92:1666–73. doi: 10.1093/jnci/92.20.1666. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Kikuchi S, Tamakoshi K, et al. Active smoking, passive smoking, and breast cancer risk: findings from the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J Epidemiol. 2008;18:77–83. doi: 10.2188/jea.18.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirie K, Beral V, Peto R, Roddam A, Reeves G, Green J. Passive smoking and breast cancer in never smokers: prospective study and meta-analysis. Int J Epidemiol. 2008;37:1069–79. doi: 10.1093/ije/dyn110. [DOI] [PubMed] [Google Scholar]
- 20.Lash TL, Aschengrau A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res Treat. 2002;75:181–4. doi: 10.1023/a:1019625102365. [DOI] [PubMed] [Google Scholar]
- 21.Morabia A, Bernstein M, Heritier S, Khatchatrian N. Relation of breast cancer with passive and active exposure to tobacco smoke. Am J Epidemiol. 1996;143:918–28. doi: 10.1093/oxfordjournals.aje.a008835. [DOI] [PubMed] [Google Scholar]
- 22.Gammon MD, Eng SM, Teitelbaum SL, et al. Environmental tobacco smoke and breast cancer incidence. Environ Res. 2004;96:176–85. doi: 10.1016/j.envres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Kropp S, Chang-Claude J. Active and passive smoking and risk of breast cancer by age 50 years among German women. Am J Epidemiol. 2002;156:616–26. doi: 10.1093/aje/kwf093. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KC, Hu J, Mao Y. Passive and active smoking and breast cancer risk in Canada, 1994-97. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control. 2000;11:211–21. doi: 10.1023/a:1008906105790. [DOI] [PubMed] [Google Scholar]
- 25.Smith SJ, Deacon JM, Chilvers CE. Alcohol, smoking, passive smoking and caffeine in relation to breast cancer risk in young women. UK National Case-Control Study Group. Br J Cancer. 1994;70:112–9. doi: 10.1038/bjc.1994.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shrubsole MJ, Gao YT, Dai Q, et al. Passive smoking and breast cancer risk among non-smoking Chinese women. Int J Cancer. 2004;110:605–9. doi: 10.1002/ijc.20168. [DOI] [PubMed] [Google Scholar]
- 27.Sandler DP, Everson RB, Wilcox AJ. Passive smoking in adulthood and cancer risk. Am J Epidemiol. 1985;121:37–48. doi: 10.1093/oxfordjournals.aje.a113980. [DOI] [PubMed] [Google Scholar]
- 28.Morabia A, Bernstein MS, Bouchardy I, Kurtz J, Morris MA. Breast cancer and active and passive smoking: the role of the N-acetyltransferase 2 genotype. Am J Epidemiol. 2000;152:226–32. doi: 10.1093/aje/152.3.226. [DOI] [PubMed] [Google Scholar]
- 29.Delfino RJ, Smith C, West JG, et al. Breast cancer, passive and active cigarette smoking and N-acetyltransferase 2 genotype. Pharmacogenetics. 2000;10:461–9. doi: 10.1097/00008571-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Millikan RC, Pittman GS, Newman B, et al. Cigarette smoking, N-acetyltransferases 1 and 2, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:371–8. [PubMed] [Google Scholar]
- 31.Marcus PM, Newman B, Millikan RC, Moorman PG, Baird DD, Qaqish B. The associations of adolescent cigarette smoking, alcoholic beverage consumption, environmental tobacco smoke, and ionizing radiation with subsequent breast cancer risk (United States) Cancer Causes Control. 2000;11:271–8. doi: 10.1023/a:1008911902994. [DOI] [PubMed] [Google Scholar]
- 32.Bonner MR, Nie J, Han D, et al. Secondhand smoke exposure in early life and the risk of breast cancer among never smokers (United States) Cancer Causes Control. 2005;16:683–9. doi: 10.1007/s10552-005-1906-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Wu K, Lin X, et al. Passive Smoking and Other Factors at Different Periods of Life and Breast Cancer Risk in Chinese Women who have Never Smoked - A Case-control Study in Chongqing, People's Republic of China. Asian Pac J Cancer Prev. 2000;1:131–7. [PubMed] [Google Scholar]
- 34.Roddam AW, Pirie K, Pike MC, et al. Active and passive smoking and the risk of breast cancer in women aged 36-45 years: a population based case-control study in the UK. Br J Cancer. 2007;97:434–9. doi: 10.1038/sj.bjc.6603859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slattery ML, Curtin K, Giuliano AR, et al. Active and passive smoking, IL6, ESR1, and breast cancer risk. Breast Cancer Res Treat. 2008;109:101–11. doi: 10.1007/s10549-007-9629-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollison DE, Brownson RC, Hathcock HL, Newschaffer CJ. Case-control study of tobacco smoke exposure and breast cancer risk in Delaware. BMC Cancer. 2008;8:157. doi: 10.1186/1471-2407-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahern TP, Lash TL, Egan KM, Baron JA. Lifetime tobacco smoke exposure and breast cancer incidence. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9376-1. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds P, Goldberg DE, Hurley S. Prevalence and patterns of environmental tobacco smoke exposures among California teachers. Am J Health Promot. 2004;18:358–65. doi: 10.4278/0890-1171-18.5.358. [DOI] [PubMed] [Google Scholar]
- 39.Johnson KC. Accumulating evidence on passive and active smoking and breast cancer risk. Int J Cancer. 2005;117:619–28. doi: 10.1002/ijc.21150. [DOI] [PubMed] [Google Scholar]
- 40.Miller MD, Marty MA, Broadwin R, et al. The association between exposure to environmental tobacco smoke and breast cancer: a review by the California Environmental Protection Agency. Prev Med. 2007;44:93–106. doi: 10.1016/j.ypmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Lee PN, Hamling J. Environmental tobacco smoke exposure and risk of breast cancer in nonsmoking women: a review with meta-analyses. Inhal Toxicol. 2006;18:1053–70. doi: 10.1080/08958370600945432. [DOI] [PubMed] [Google Scholar]
- 42.Lash TL, Aschengrau A. Active and passive cigarette smoking and the occurrence of breast cancer. Am J Epidemiol. 1999;149:5–12. doi: 10.1093/oxfordjournals.aje.a009727. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Shi Z, Liu L. Matched case-control study for detecting risk factors of breast cancer in women living in Chengdu. Zhonghua Liu Xing Bing Xue Za Zhi. 1999;20:91–4. [PubMed] [Google Scholar]
- 44.Ambrosone CB, Kropp S, Yang J, Yao S, Shields PG, Chang-Claude J. Cigarette smoking, N-acetyltransferase 2 genotypes, and breast cancer risk: pooled analysis and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:15–26. doi: 10.1158/1055-9965.EPI-07-0598. [DOI] [PubMed] [Google Scholar]
- 45.Lissowska J, Brinton LA, Zatonski W, et al. Tobacco smoking, NAT2 acetylation genotype and breast cancer risk. Int J Cancer. 2006;119:1961–9. doi: 10.1002/ijc.22044. [DOI] [PubMed] [Google Scholar]
- 46.Chang-Claude J, Kropp S, Jager B, Bartsch H, Risch A. Differential effect of NAT2 on the association between active and passive smoke exposure and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:698–704. [PubMed] [Google Scholar]
- 47.Lilla C, Risch A, Kropp S, Chang-Claude J. SULT1A1 genotype, active and passive smoking, and breast cancer risk by age 50 years in a German case-control study. Breast Cancer Res. 2005;7:R229–37. doi: 10.1186/bcr976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarty KM, Santella RM, Steck SE, et al. PAH-DNA adducts, cigarette smoking, GST polymorphisms, and breast cancer risk. Environ Health Perspect. 2009;117:552–8. doi: 10.1289/ehp.0800119. [DOI] [PMC free article] [PubMed] [Google Scholar]