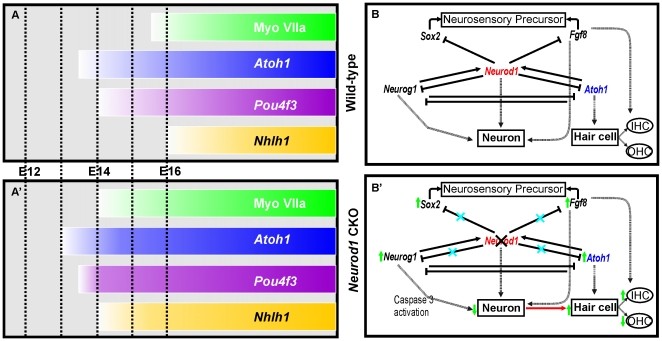
Figure 9. Neurod1 regulates neuronal differentiation by suppression of premature hair cell differentiation of neurons possibly interacting with several target genes.
In the absence of Neurod1 several hair cell specific genes such as Myo VIIa, Atoh1, Pou4f3 and Nhlh1 are prematurely expressed with an inverse gradient of apex-to-base progression of hair cell differentiation instead of usual base-to-apex progression (cochlear expression shown with bars in A, A’). In addition, these genes are also expressed ectopically in the differentiating vestibular ganglia near the utricle and saccule. This substantial alteration of gene expression changes the organization of the apical part of the cochlea and results in the formation of ‘intragangliionic hair cells’. Our data and those of others suggest the following interaction of Neurod1 with Neurog1, Atoh1, Sox2 and Fgf8 to regulate inner ear cellular identity (B, B’). We propose that after early and transient activation of Neurod1 by Neurog1 and Atoh1 to differentiate neuron, Neurod1 suppresses Neurog1 to inhibit precursor proliferation and Atoh1 to inhibit hair cell differentiation in neurons. These three way interactions result in formation of neurons with delayed hair cell differentiation. Cross inhibition of Neurog1 and Atoh1 was previously suggested [17, 20] and we suggest that Neurod1 is a key intermediary player. Neurod1 also regulates other cell fate determining genes like Sox2 and Fgf8 which may more directly related to the observed cell fate switch. We suggest that Neurod1 deletion in early embryos disrupts this gene network and, as a consequence, the coordinated sequential neurosensory development of inner ear resulting in the transformation of some surviving neurons into ‘intraganglionic hair cells’ and alteration of the cell type specific differentiation of outer hair cells in the cochlea.