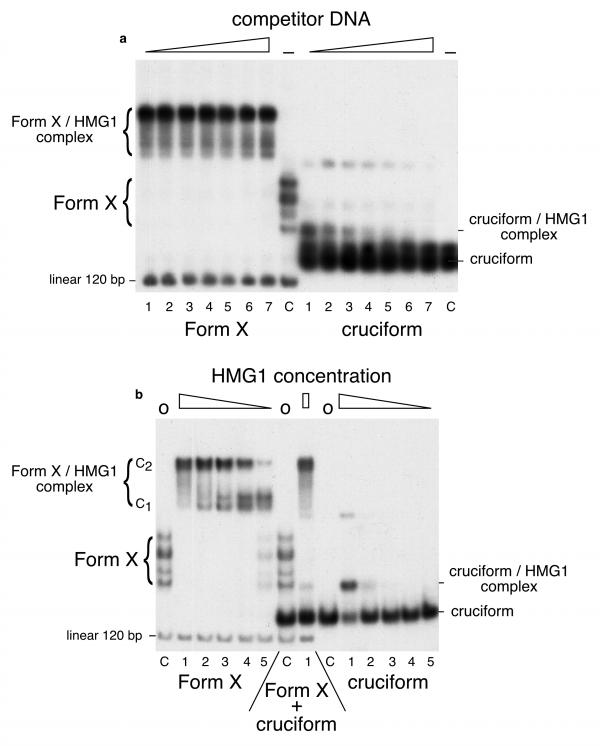
Figure 2.
Comparison of the interactions of HMG1 with Form X and cruciform. The concentrations used were: Form X: 3.5 × 10-11 M ; cruciform: 1.5 × 10-10 M ; undiluted HMG1: 3.2 × 10-8 M. (A) Form X (left) and cruciform (right) were labelled and incubated with a constant amount of HMG1 in the presence of increasing amounts of unlabelled E. coli competitor DNA. Lanes C are controls with no protein added. It is observed that Form X is entirely bound by HMG1 at all the competitor concentrations used. In contrast, cruciform is only partially bound in the first sample, and the amount of complex decreases quickly when the amount of competitor DNA is increased. Also note that linear DNA in its regular double-stranded form is not bound at all. (B) labelled Form X and cruciform were incubated in the presence of decreasing amounts of protein HMG1, with no addition of competitor DNA. Lanes C contain controls with no protein added. The protein amounts are 10, 2, 0.4, 0.08, and 0.016 ng in lanes 1 to 5, respectively. Both central samples contain a mixture of Form X plus cruciform. To better compare the results with data published in the literature, the interactions and electrophoreses of the experiments shown in this Figure were strictly done under the conditions used in [26], 6.5% polyacrylamide gels in Tris-borate buffer, resulting in a change in mobility of Form X and of Form X-HMG1/2 complex as compared to experiments of Figures 1, 3 and 4, which were done in 4% polyacrylamide gels in Tris-acetate buffer. All the experiments shown here were also performed with HMG2, with identical results.