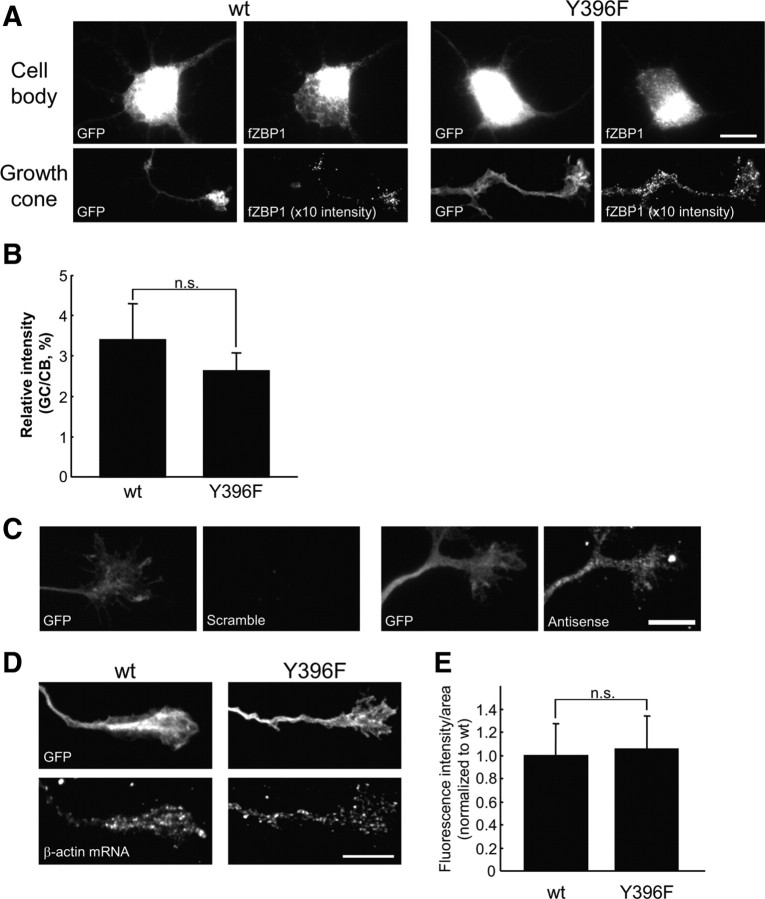
Figure 3.
ZBP1 Y396F phospho-mutant does not impair β-actin mRNA localization in growth cones. A, Localization of WT and Y396F mutant forms of flag-tagged mCherry–chick ZBP1 (fZBP1) in cell bodies and growth cones. Both WT and Y396F forms of fZBP1 were cotransfected into cortical neurons with GFP as a morphologic marker. Only growth cones >100 μm distal from the cell body were selected and analyzed. Cell body and growth cone images were acquired with the same exposure time. The fluorescence intensity of growth cone images from cells transfected with WT or mutant ZBP1 were both enhanced 10 times from the original images for improved visualization. Scale bar, 10 μm. B, Quantification of fluorescence intensities of the flag-tagged ZBP1 signals in growth cones using IF with anti-flag antibody. Relative intensity is represented as a ratio of fluorescence intensity in a growth cone (GC) to that in a cell body (CB). Data are shown as mean ± SEM (n = 10). n.s., Not significantly different using Student's t test. C, FISH of β-actin mRNA using antisense and scrambled probes (right). Note that scrambled probes did not detect a significant signal. GFP expression in the same growth cone is shown (left). D, In situ hybridization for β-actin mRNA in growth cones of cortical neurons transfected with wild-type or Y396F mutant of fZBP1 (bottom). GFP was cotransfected as a morphologic marker (top). β-Actin mRNA was detected using three oligonucleotide probes labeled with DIG and was visualized with anti-DIG antibody. Scale bar, 10 μm. E, Quantification of fluorescence intensities of β-actin mRNA signals in growth cones under the same treatments as in D. Data are indicated as mean ± SEM (n = 10). No significant difference in β-actin mRNA levels between neurons that express wild-type or Y396F mutant was apparent using a Student's t test.