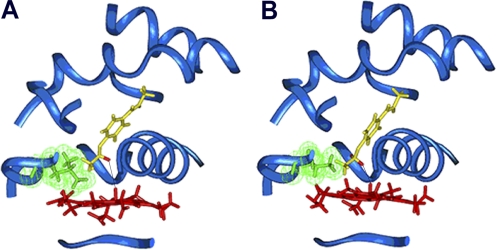
Fig. 1.
Binding orientation of phenacetin within the active site of CYP1A2 WT (A) and the L382V mutant (B). The protein backbone is depicted as a blue ribbon; the side chain of residue 382 is green, with van der Waals surface displayed; heme is red; and phenacetin is yellow, with hydrogens at the oxidation site shown in red. The distance between the hydrogen to be abstracted and the ferryl oxygen (marked with a black line) is 3.6 Å in the WT and 2.9 Å in the L382V mutant.