Figure 4. The folding status of an antibody domain controls IgG secretion in vivo.
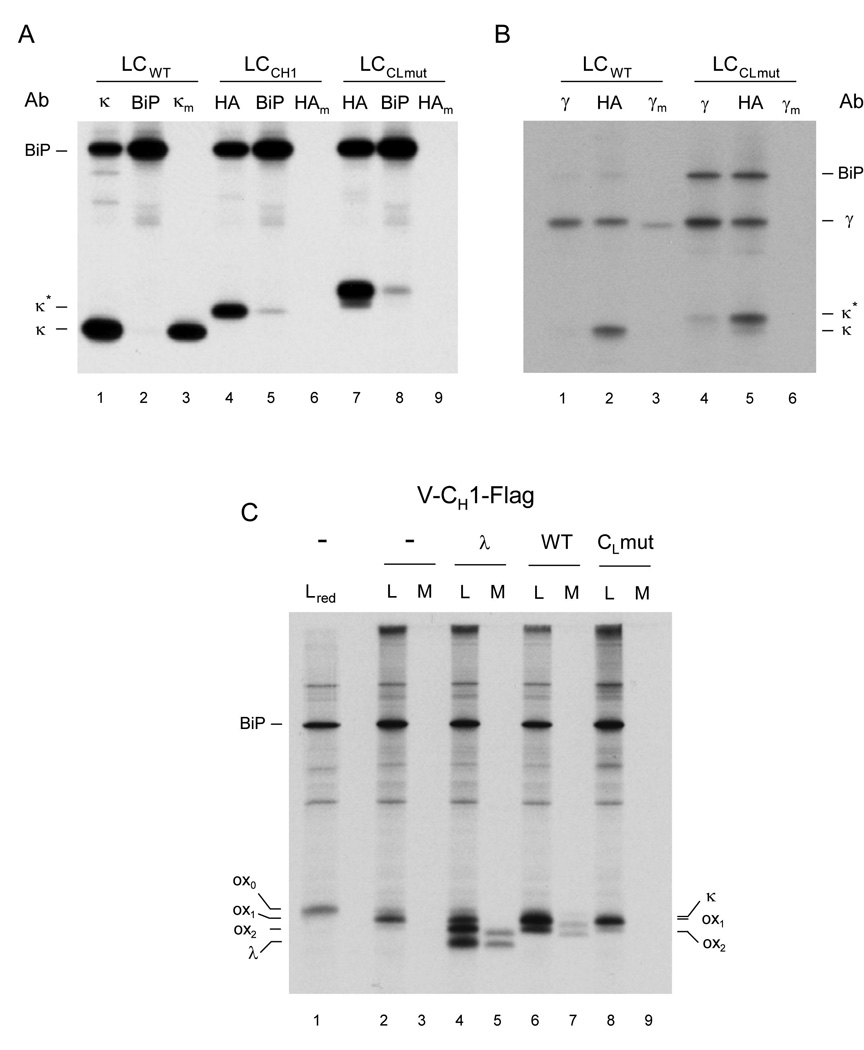
(A) COS-1 cells were co-transfected with vectors encoding BiP and either a wild type light chain (LCwt), a light chain containing the CH1 domain instead of the CL domain (LCCH1), or a light chain containing an unfolded CL domain (LCCLmut). Cells were metabolically labeled for 3 h and both cell lysates (no subscript) and culture supernatants (subscript m) were immunoprecipitated with the indicated antisera (Ab). Precipitated proteins were separated by SDS-PAGE under reducing conditions and visualized by autoradiography. (B) COS-1 cells were co-transfected as in (A) except that a vector encoding a chimeric humanized mouse heavy chain was also included. Cells were labeled and analyzed as in (A). (C) COS-1 cells were co-transfected with vectors encoding BiP, a Flag-tagged truncated heavy chain consisting of only the VH and CH1 domains (Lee et al., 1999), and with the indicated light chain constructs (i.e., λ, wild type κ, or CL mutant κ). Cells were metabolically labeled and both cell lysates (L) and culture supernatants (M) were immunoprecipitated with the anti-Flag antibody. Precipitated proteins were separated by SDS-PAGE under non-reducing condition (except the first lane, which included 2-ME in the sample buffer and is indicated as red) and visualized by autoradiography. Mobilities of completely reduced (ox0), partially oxidized (ox1), and fully oxidized (ox2) forms of truncated heavy chain, as well as those of λ and κ light chains are indicated. The tagged forms of the κ light chain constructs co-migrate with the ox1 form of the truncated heavy chain.
