Abstract
Although topical drug delivery is a convenient route of administration to treat various eye diseases, it has serious limitations due to rapid clearance of the formulation from the surface of the eye. In this study, we engineered microparticles for both sustained drug delivery and prolonged residence time on the extraocular surface. Microparticles were fabricated by emulsification using poly(lactic-co-glycolic acid) (PLG) and poly(ethylene glycol) (PEG) as the core material and mucoadhesion promoter, respectively. The particle size was controlled to be less than 10 µm to avoid eye irritation and for eventual clearance through the lacrimal canals. In vitro mucoadhesion tests showed that PLG microparticles with PEG adhered better to the mucous membrane under the conditions employed in this study compared to the microparticles without PEG. When an aqueous suspension of microparticles with PEG was administered topically to the rabbit eye in vivo, microparticles were seen for up to 30 min on the ocular surface in the cul-de-sac, which was a dramatic increase in residence time as compared to conventional eye drop formulations. We conclude that mucoadhesive microparticles are promising vehicles for ophthalmic drug delivery.
Keywords: A. polymers, C. electron microscopy, D. microstructure, D. surface properties
1. Introduction
Topical drug delivery has been widely applied to treat diseases of the eye, but is limited by low drug bioavailability due to rapid clearance by tear drainage and absorption into the conjunctiva [1]. This limitation could be addressed by a drug delivery system that can provide sustained drug release as well as reside on the surface of the eye for a prolonged period. This could be accomplished using well-known technology by encapsulating drug for slow release from biocompatible microparticles, which provide an attractive form of drug delivery vehicle due to their ease of fabrication, simplicity of administration, and possible use in localized and targeted delivery [2]. However, the short residence time of conventional microparticles on the ocular surface caused by tear drainage could limit the utility of slow release microparticles for therapy of ocular disease.
In this work, we examine the hypothesis that mucoadhesive microparticles may offer a means for slow drug release from microparticles that remain adherent to the ocular surface for an extended time [3]. We therefore designed microparticles made of a blend of poly(lactic-co-glycolic acid) and poly(ethylene glycol), which are employed as the core material [4] and mucoadhesiveness promoter [5], respectively. The resulting particles should exhibit sustained release properties with reduced loss caused by tear drainage, hence increasing drug bioavailability. The size of the microparticles was also controlled to be less than 10 µm to avoid possible eye irritation [6] and for eventual clearance through the lacrimal canals, which are 300 – 500 µm in diameter.
2. Experimental
Microparticle fabrication
Microparticles were fabricated by emulsification. Briefly, a polymer solution was prepared by dissolving 200 mg PLG (PLG, i.v. = 0.17 – 0.25 dL/g), 20 mg PEG (PEG, M.W. = 6000) and 2 mg of either rhodamine B or Nile Red in 2 ml methylene chloride, which was then dispersed in 15 ml water containing polyvinyl alcohol (2% w/v) and sonicated to obtain emulsion droplets of the appropriate size (100 W, 7 s; CV33 ultrasonic converter with VC505 power supply, Sonics & Materials). The emulsion was stirred overnight to evaporate the methylene chloride and obtain solid microparticles. The resulting microparticles were filtered to remove the particles larger than 10 µm. Microparticle size distribution was measured using a Beckman Multisizer 3 Coulter Counter.
In vitro mucoadhesion test
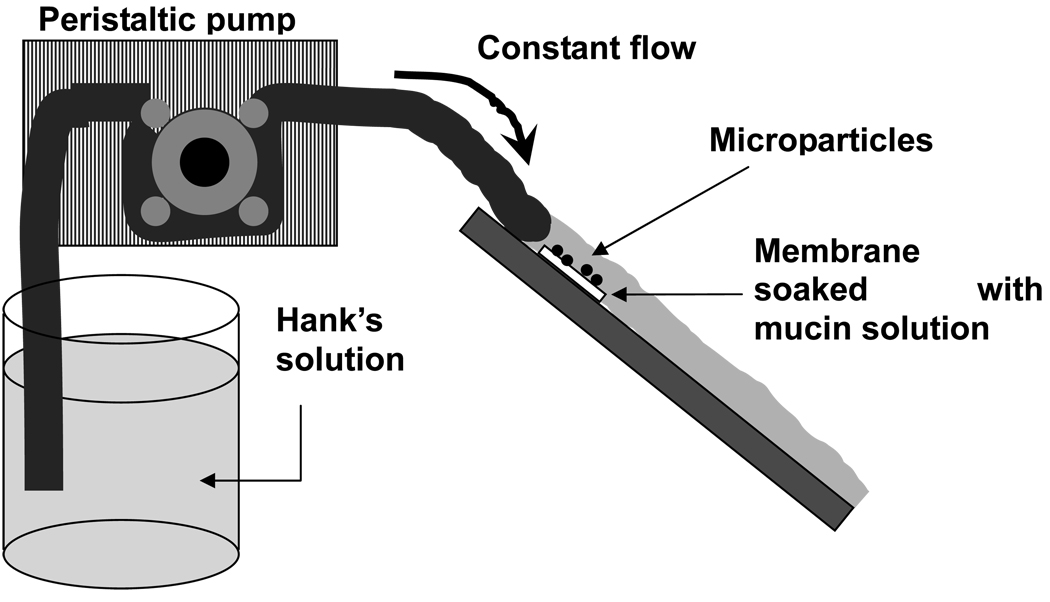
Mucoadhesiveness of the microparticles was examined using the apparatus shown in Fig. 1. A hydrophilic membrane (cellulose nitrate membrane, 0.45 µm pore size) was first soaked for 2 h in an aqueous mucin solution (0.1% mucin from porcine stomach, Type II). Then, 20 µl of a 10 mg/ml suspension of either PLG microparticles or PLG/PEG microparticles labeled with rhodamine was applied as a single drop at the center of the membrane. The membrane was then immediately washed with a continuous flow of Hank’s solution for 5 min at a rate of 10 ml/min. The number of remaining particles was measured with both fluorescence spectroscopy and microscopy.
Figure 1.
Schematic drawing of the apparatus for the mucoadhesion test. Rhodamine B-loaded microparticles were applied to a mucous membrane and Hank’s solution was flowed over the membrane to roughly simulate tear fluid flow over the ocular surface.
In vivo mucoadhesion test
Retention of PLG/PEG microparticles on the surface of the eye was also examined in vivo. A 5 µl aqueous suspension of Nile Red-labeled microparticles was formulated (20 mg/ml) and administered topically to the rabbit eye as a single drop applied to the center of the cornea. The rabbit was anesthetized to control blinking (which was carried out manually by the investigators once per 5 min) and obtain still images from the eye.
3. Results and Discussion
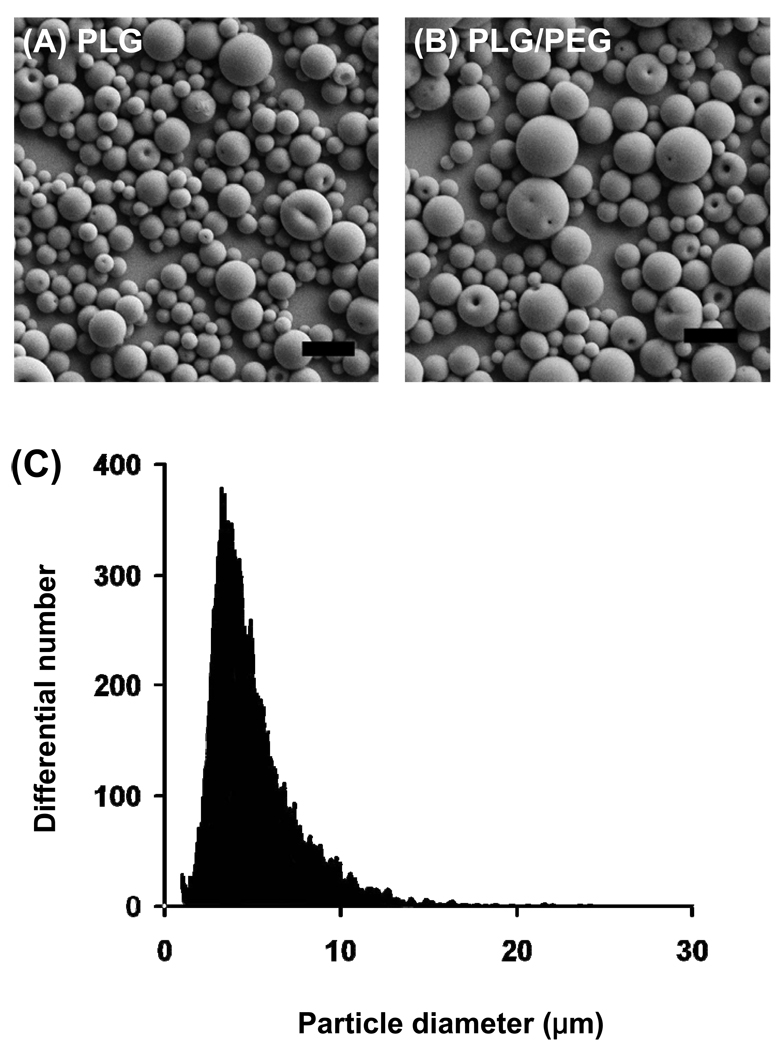
Microparticles were fabricated out of only PLG (Fig. 2A) and out of a blend of PLG and PEG (Fig. 2B). Because PEG should act as a mucoadhesion promoter, these microparticles allowed us to compare the effect of mucoadhesion on the lifetime of microparticles on the ocular surface. For both types of microparticles, a similar spherical shape and smooth morphology were observed. The particle size distribution was well controlled and exhibited diameters generally smaller than 10 µm (Fig. 2C).
Figure 2.
Microparticles prepared for ocular adhesion studies. (A) PLG microparticles. (B) PLG/PEG microparticles. (C) Size distribution of a representative batch of microparticles. The scale bars = 10 µm.
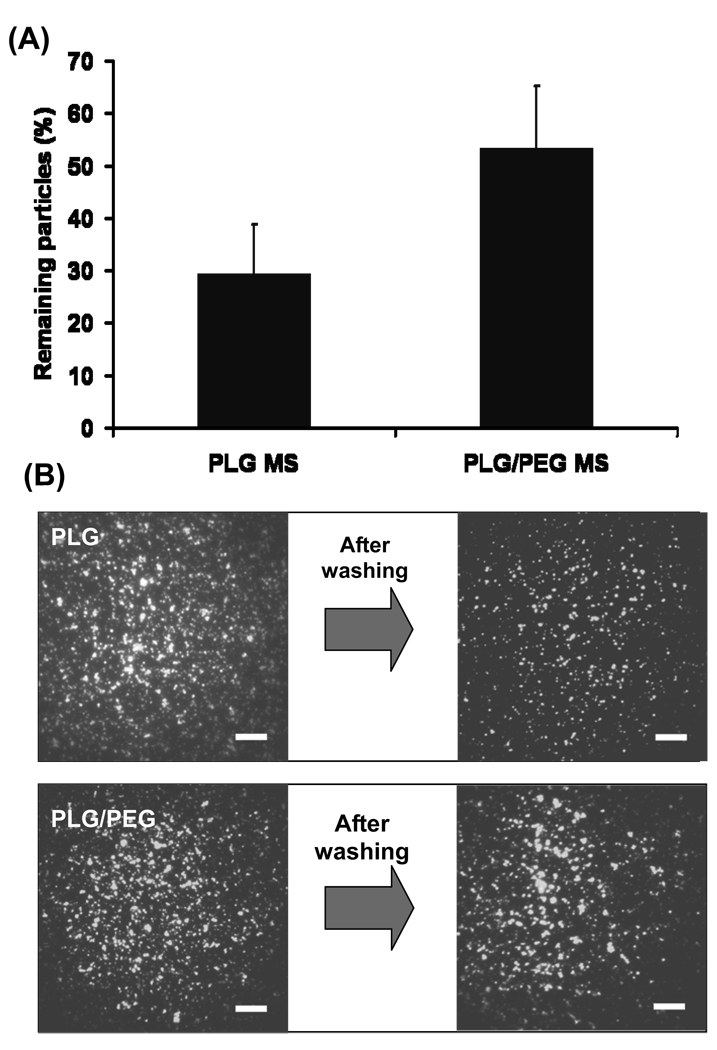
Mucoadhesion of microparticles was examined in vitro using a membrane soaked with mucin to roughly simulate the ocular surface (Fig. 1). Particles applied on the mucous membrane were exposed to flowing Hank’s solution to roughly simulate tear fluid flow. Under these conditions, 29% of PLG microparticles remained adherent, whereas 53% of PLG/PEG microparticles remained adherent (Fig. 3A). This shows that PLG/PEG microparticles adhered better to the mucous layer than PLG microparticles (Student’s t-test, p < 0.05). Fluorescence microscopy of the mucous membranes yielded images consistent with this quantitative analysis (Fig. 3B).
Figure 3.
In vitro mucoadhesion of microparticles. (A) Percentage of microparticles remaining on the mucous membrane after rinsing. (B) Fluorescence images of PLG and PLG/PEG microparticles on the mucous membrane before and after rinsing. The scale bars = 500 µm.
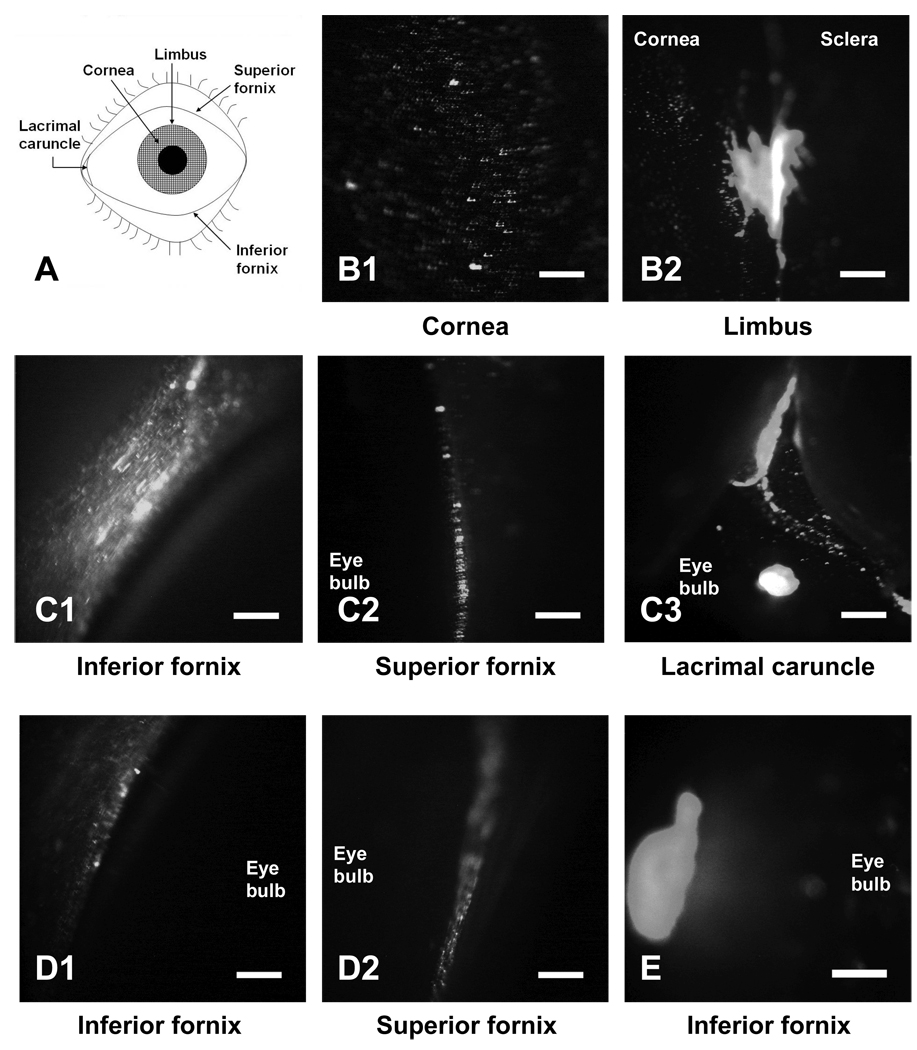
Because PLG/PEG microparticles exhibited good mucoadhesion, we assessed the retention of these particles on the ocular surface of the rabbit eye in vivo (Fig. 4). Immediately after administration, some of the microparticles were observed on the cornea (Fig. 4B1). However, most particles from this liquid formulation accumulated in the valley present near the limbus (Fig. 4B2). After 5 min, the rabbit was forced to blink and the particle distribution was again imaged. This caused most microparticles to be removed from the cornea, such that most collected at the inferior and superior fornix under the lower and upper eyelids, respectively (Figs. 4C1 and 4C2), and in the lacrimal caruncle, where the tear fluid drains from the eye (Fig. 4C3).
Figure 4.
In vivo mucoadhesion of microparticles to the rabbit eye. (A) Schematic drawing of ocular anatomy. Regions of the ocular surface imaged (B) immediately after administration, (C) 5 min after administration, (D) after washing with ~50 µl of saline, and (E) 30 min after administration. The scale bars = 500 µm.
A drop of saline (~50 µl) was then placed on the eye surface, which washed away most of the particles in the lacrimal caruncle, but many microparticles remained in the upper and lower fornix (Fig. 4D). At 30 min after administration, the remaining microparticles were observed as a single entangled mass in the lower fornix. The persistence of microparticles on the ocular surface for 30 min is remarkable, as compared to the 3 – 7 min lifetime of conventional eye drop formulations [7].
4. Conclusion
Microparticles with prolonged residence time on the extraocular surface were engineered for ophthalmic drug delivery. PLG and PEG were utilized as core material and mucoadhesion promoter, respectively. PLG/PEG microparticles adhered to a model mucous membrane in vitro better than PLG particles under the conditions tested. When a microparticle suspension was applied topically to the rabbit eye, microparticles first flowed to the valley formed near the limbus and then rapidly drained to the lacrimal caruncle, where the tear fluid drains from the eye, and to the superior and inferior fornix found under the upper and lower eyelids, respectively. Many microparticles remained there for 30 min, which is much longer than the residence time of conventional eye drops. Therefore, microparticles formed of a PLG core material and a PEG mucoadhesion promoter are promising vehicles to achieve sustained drug delivery on the surface of the eye to treat ocular disorders with improved drug bioavailability.
Acknowledgement
We thank Henry Edelhauser, Glenn Holly, Yeu Chun Kim, Bernard McCarey and Samir Patel for technical assistance and helpful discussions. This research was partially funded by the National Institutes of Health.
References
- 1.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 2.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 3.Chowdary KP, Rao YS. Mucoadhesive microspheres for controlled drug delivery. Biol Pharm Bull. 2004;27:1717–1724. doi: 10.1248/bpb.27.1717. [DOI] [PubMed] [Google Scholar]
- 4.Gavini E, Chetoni P, Cossu M, Alvarez MG, Saettone MF, Giunchedi P. PLGA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: in vitro/in vivo studies. Eur J Pharm Biopharm. 2004;57:207–212. doi: 10.1016/j.ejpb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga K, Ono S, Narioka K, Kakemi M, Morimoto K, Yamashita S, Namba Y, Oku N. Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome's surface on the GI transit of insulin. J Pharm Sci. 1999;88:248–252. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CH, Tung SM, Lu DW, Yeh MK. In vitro and in vivo evaluation of an ocular delivery system of 5-fluorouracil microspheres. J Ocul Pharmacol Ther. 2001;17:545–553. doi: 10.1089/10807680152729239. [DOI] [PubMed] [Google Scholar]
- 7.Schoenwald RW. In: Ocular pharmacokinetics. Zimmerman TJ, editor. Lippincott-Raven Publishers; 1997. pp. 119–138. [Google Scholar]