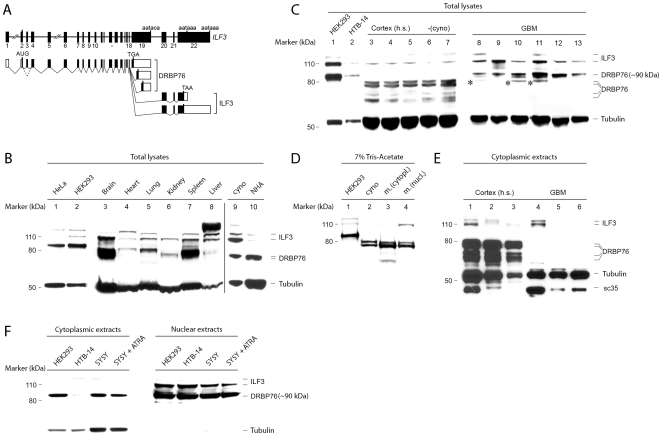
Figure 1. NFAR protein isoform- and subcellular distribution in normal and neoplastic tissues.
A. Intron-exon organization of the ILF3 gene [25], [33]. Exons are indicated by black boxes (top); alternative splicing yields diverse transcripts encoding DRBP76/ILF3 proteins with distinct C-termini (bottom; coding sequences are indicated by solid boxes). Hatched lines indicate proposed alternative splicing of exon 3 [25]. B. DRBP76 immunoblot of HeLa or HEK293 cell lysates (lanes 1–2) or non-fractionated murine tissue extracts of the indicated origin (lanes 3–8). (Lanes 9–10) DRBP76 immunoblot of normal Cynomologus brain (cyno) and NHA cell lysate. C. DRBP76 immunoblots of HEK293 and HTB-14 glioma cell lysates (lanes 1, 2) and total extracts prepared from normal cortex (3, 6 = frontal; 4, 7 = temporal; 5 = parietal) of human (h.s.) or simian (cyno) brain or human GBM tissue samples (8–13). Asterisks indicate histopathologically confirmed contamination with normal CNS. D. NFAR protein immunoblot analyzed with 7% tris-acetate gel electrophoresis. Simian (cyno) and fractionated cytoplasmic and nuclear murine (m.) brain extracts were studied in parallel with HEK293 lysates. Protein bands are labeled in (E). E. Evaluation of NFAR proteins in fractionated, cytoplasmic extracts from human normal brain and GBM tissues. Immunoblot filters were deliberately over-exposed to reveal all DRBP76-immunoreactive material. sc35 is a nuclear spliceosome component. F. DRBP76 immunoblot from fractionated extracts of the indicated cell lines. SH-SY5Y (SY5Y) cells were untreated or treated to induce a neuronal phenotype with ATRA.